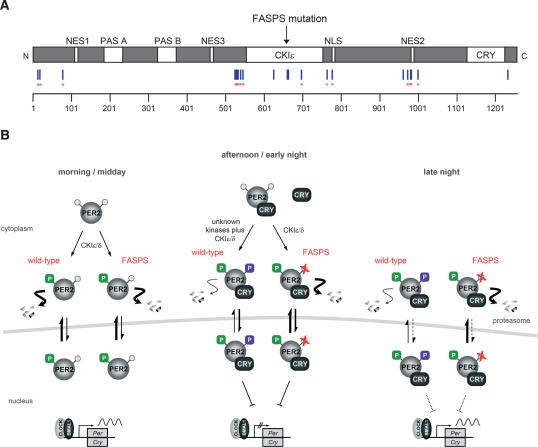
Figure 8.
Schematic representation of PER2 phosphorylation sites and their functional impact on the circadian oscillator. (A) Linear map of the PER2 protein with positions of phosphorylated residues identified by mass spectrometry and additional functionally important domains (see also Table 1). (Blue lines) Sites phosphorylated by endogenous kinases in cells; (red asterisks) sites also phosphorylated in vitro by CKIδ. Functional domains: (PAS) PER-ARNT-SIM domain; (NES) nuclear export sequence; (NLS) nuclear localization signal; (CKIε) CKIε-binding domain; (CRY) CRY1/2-binding domain. (B) Model for the differential effects of PER2 phosphorylation on circadian oscillations. PER2 contains at least two functionally different sets of phosphorylation sites—one primarily mediating proteasomal degradation (green), the other nuclear retention (purple). In FASPS-PER2 (right side of the panels), the latter cannot be phosphorylated because Ser 659 is mutated to glycine (Toh et al. 2001). At the beginning of the circadian cycle (morning/ midday), newly synthesized PER2 protein shuttles between nucleus and cytoplasm, where it is phosphorylated by kinases such as CKIε/δ at sites that target it for rapid proteasomal degradation in the cytoplasm. Later (afternoon/early night), complex formation with CRY proteins enhances the nuclear localization of the PER2–CRY complex and likely activates or recruits additional kinases to the PER2–CRY complex. These yet-unknown kinases phosphorylate PER2 at the FASPS site, which serves as a priming site for CKIε/δ phosphorylation at downstream residues. Together, this leads to nuclear accumulation of the PER2–CRY complex and thereby to transcriptional repression of CLOCK–BMAL1 transactivation. At the end of the circadian cycle (late night), the PER2–CRY repression is released because the PER2–CRY complex is degraded in the cytoplasm after nuclear export. In FASPS-PER2, however, the region responsible for nuclear retention cannot be phosphorylated (red crosses), leading to premature nuclear export of the PER2–CRY complex, and thus to an earlier cytosolic degradation and to a faster circadian cycle.