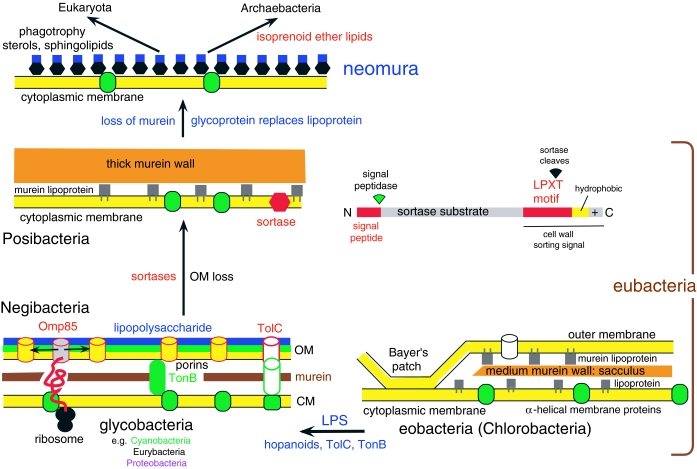
Figure 3.
Membrane evolution and the fundamental contrast between negibacteria and posibacteria. Earliest cells were negibacteria bounded by two lipoprotein membranes: an inner cytoplasmic membrane (CM) impermeable to small hydrophilic molecules (with alpha-helical membrane proteins: green) and an OM, more permeable because of large proteinaceous pores, made by porin proteins. Between the two negibacterial membranes is the murein sacculus or cell wall: a gigantic hollow bag-shaped peptidoglycan molecule covalently cross-linked in three dimensions, rigidly enough to resist high internal osmotic pressures of more than 20 atm. The simplest negibacterial envelope (right) is in Chlorobacteria, which lack Omp85 that inserts OM β-barrel proteins in glycobacteria (left) and Hadobacteria (not shown: intermediate in complexity between chlorobacteria and glycobacteria), in both of which all known OM proteins are β-barrels. In eobacteria, the CM and OM both consist of acyl ester phospholipids plus embedded proteins. Glycobacteria retained these, but also have hopanoids that make membranes more rigid, and replaced phospholipids in the OM outer leaflet by the immensely more complex lipopolysaccharide (LPS), which so dramatically reduces OM permeability that they simultaneously evolved novel protein export machinery (using the β-barrel TolC protein) and the TonB importer of small molecules (Cavalier-Smith 2006a). For clarity, lipoproteins and Bayer's patches are not shown for glycobacteria. Posibacteria lack an OM, but have a much thicker wall of murein peptidoglycan supplemented by other polymers (teichoic acids in Endobacteria; a disparate array in Actinobacteria). Murein walls can grow and divide only by cleavage of covalent bonds by murein hydrolases. The transition from negibacteria to posibacteria involved loss of the cell wall and the origin of novel families of sortase enzymes that recognize a C-terminal LPXT motif on numerous extracellular proteins which they cleave and use for attaching them covalently to the cell wall, preventing their escape as would otherwise be likely without an OM. Posibacterial wall-attached proteins include murein lipoproteins with hydrophobic lipid tails embedded in the outer leaflet of the cytoplasmic membrane, not in the OM inner leaflet as in negibacteria.