Fig. 4. The effect of missense mutations of TBX5 on the interaction between TBX5 and NKX2.5 in vivo (A) and in vitro (B).
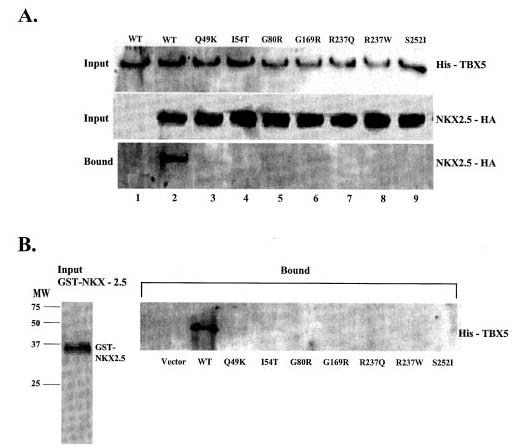
A, HeLa cells were transiently transfected with expression plasmids for His-TBX5 and NKX2.5-HA. Total cell lysates containing His-tagged TBX5 and/or HA-tagged NKX2.5 were incubated with Ni-NTA beads, separated by 12% SDS-PAGE, and analyzed by Western blot with an anti-His antibody (upper panel) or with an anti-HA monoclonal antibody (middle panel). Proteins bound to Ni-NTA beads were washed with washing buffer, eluted, and fractionated by 12% SDS-PAGE and analyzed by Western blot with anti-mouse HA for NKX2.5. Lane 1, wild type (WT) TBX5 without co-transfection of NKX2.5; lane 2, wild type TBX5 with co-transfection of NKX2.5; lanes 2–9, TBX5 with Q49K, I54T, G80R, G169R, R237Q, R237W, and S252I co-transfected with NKX2.5-HA, respectively. B, GST-NKX2.5 fusion protein and in vitro-translated His-tagged TBX5 were incubated with glutathione-Sepharose 4B beads, and proteins bound to the beads were washed with PBS, eluted, fractionated by 12% SDS-PAGE, and analyzed by Western blot with an anti-His antibody for detecting TBX5. Approximately equal amounts of wild type and various mutant TBX5 proteins were used in the GST pull-down assay as shown in the legend to Fig. 2B.
