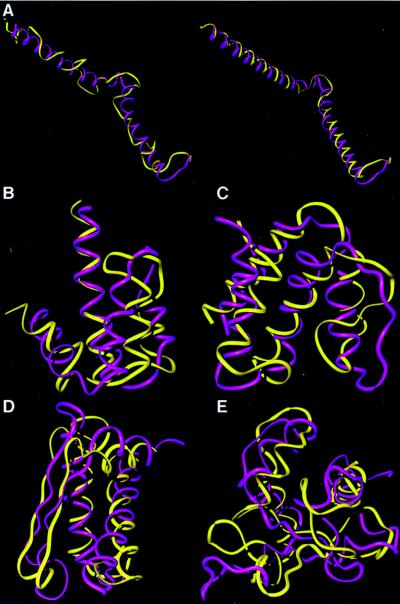
Figure 3.
Comparisons of the native conformations (purple) with their topomeric counterparts from the generic structure sets (yellow). To facilitate viewing, the local geometry of each generic conformation has been refined to incorporate native helix and β-strand segments while preserving the tertiary fold topology. This refinement is demonstrated in a, where the generic structure (left, in yellow) is refined by using the native helix assignment (right, in yellow). (a) The 65-residue segment from the NMR determined structure of the proteolytic fragment from Bacteriorhodopsin (44) (1bct). This example is one of many semicompact test folds that was topomerically matched by a GP structure. Thus, our estimate considers semicompact as well as compact topomers. (b) A 65-residue Porcine C5adesArg (1c5a) (45). (c) An 80-residue fragment from acyl-CoA binding protein (1aca) (46). (d) An 80-residue segment from domain four of the N-terminal domain of 70-kDa heat-shock cognate protein (1hpm04) (47). (e) A 100-residue segment from heat shock transcription factor (1hks) (48).