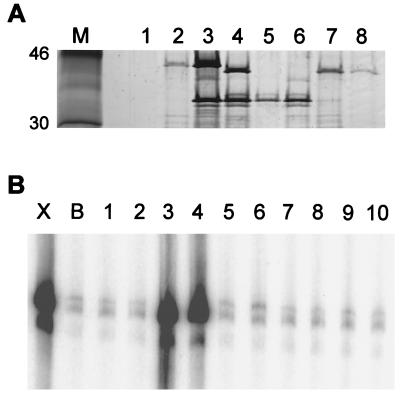
Figure 2.
Metal-affinity binding and activity of candidate intermediate transcription factors expressed in bacteria. (A) Histidine-tagged proteins were partially purified by metal-affinity chromatography. A sample (20 μl) of each 0.1 M imidazole fraction was analyzed on a 4–20% polyacrylamide gel, and proteins were detected by silver staining. Marker proteins are shown in lane M. The expression plasmids contained no insert (lane 1); HisA23R (lane 2); A8R and HisA23R (lane 3); HisA8R and A23R (lane 4); HisA8R (lane 5); HisA8R and F11L (lane 6); A8R and HisF11L (lane 7); or HisF11L (lane 8). The positions of 46- and 30-kDa marker proteins are shown (Left). (B) the partially purified proteins were tested for transcription factor activity as described in the legend to Fig. 1. Lanes 1–8, 17 μl of dialyzed imidazole fraction corresponding to lanes of the same number in A; lane 9, 5 μl each of proteins used in lanes 2 and 5; lane 10, 8.5 μl each of proteins used in lanes 2 and 5; lane X, partially purified VITF-X; lane B, buffer control.