Figure 1. Structure of Vps26A.
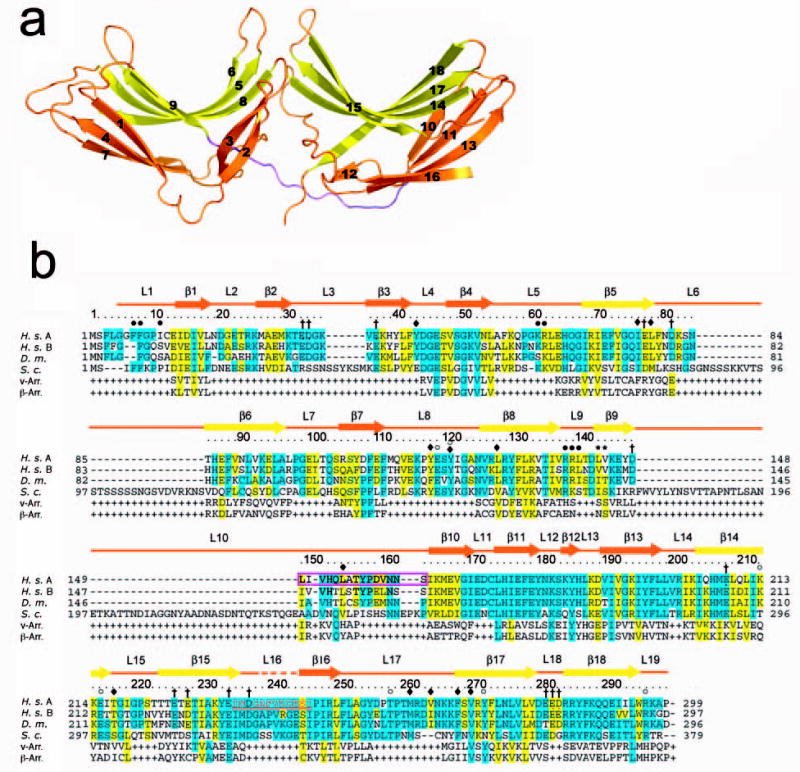
(a) Ribbon model of Vps26. Inner β-strands are colored in yellow and outer β-strands are colored in orange. The interdomain linker is colored in magenta. All structural figures were generated with Pymol (W. Delano, www.pymol.org). (b) Structure-based sequence alignment of Vps26 orthologs and arrestins (visual arrestin PDB: 1CF1 and β-arrestin 1 PDB: 1G4M). Residues involved in Vps35 binding are colored in red; polar core residues are labeled by open circles ○; hydrophobic residues in the interdomain contact are labeled by filled diamonds♦; acidic patch residues are labeled by daggers †; basic patch residues are labeled by filled circles•; yeast dominant negative mutants are labeled with an asterisk *; and the N-C domain linker is boxed in magenta.
