Figure 4. Structural similarities between Vps26 and β-arrestin.
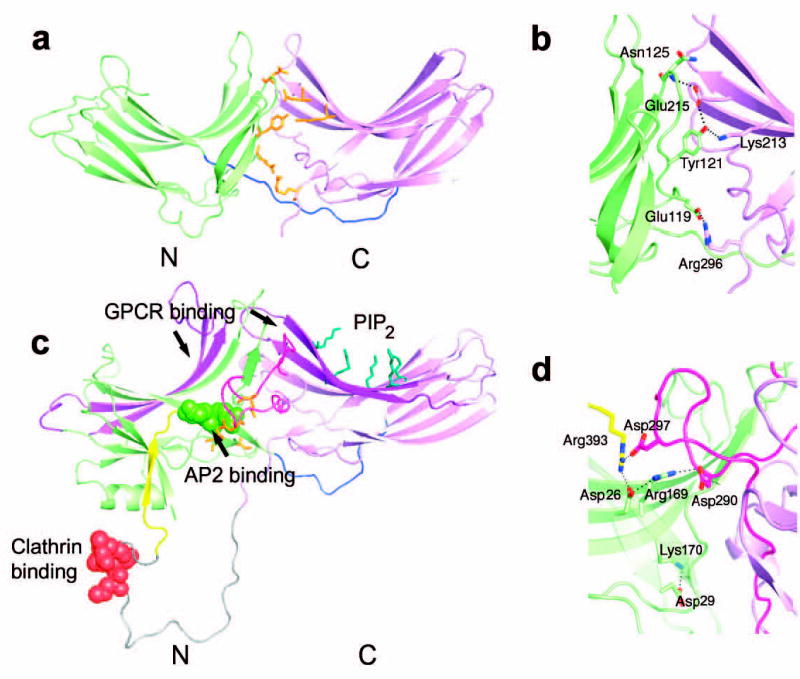
(a) Vps26 is shown in a ribbon model with the N domain colored in lime and the C domain in violet. The interdomain linker is colored blue. Polar core residues are colored in orange. (b) β -arrestin1 is shown in a ribbon model in the same color scheme as (a). The disordered region at the C tail is modeled with in an arbitrary conformation and colored in grey with the clathrin binding site shown in red spheres. The AP-2 binding site is shown in green spheres. The GPCR binding sites in the N and C domain cups are colored in purple. The PIP2 binding residues are shown in blue balls and sticks. The C domain lariat and the C terminal tail are colored in pink and yellow respectively. (c) Polar core residues in Vps26. (d) Polar core residues in β-arrestin1.
