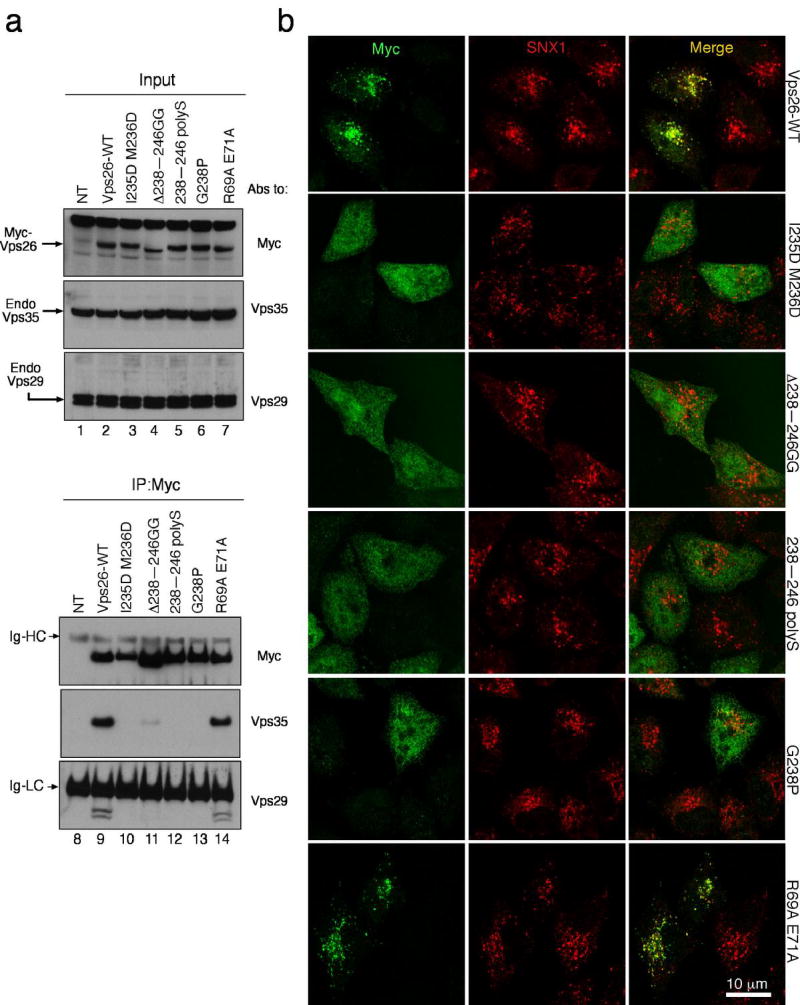
Figure 6. The binding site for Vps35 is required for Vps26 integration into the retromer complex in vivo.
(a) Lysates from HeLa cells that were either not transfected (lanes 1 and 8, NT) or transfected with cDNAs encoding wild-type (lanes 2 and 9), IM-235,236-DD (lanes 3 and 10), Δ238–246 GG (lanes 4 and 11), 238–246 polyS (lanes 5 and 12), G238P (lanes 6 and 13) and R69A E71A (lanes 7 and 14) forms of myc-tagged Vps26 were subjected to immunoprecipitation (IP) using a mouse monoclonal antibody to the myc epitope The lysates (1% of the total, lanes 1–7) and immunoprecipitates (lanes 8–14) were subsequently analyzed by SDS-PAGE and immunoblotting (IB) with antibodies to the myc epitope (top panel), Vps35 (middle panel) or Vps29 (bottom panel). (b) The intracellular localization of Vps26-myc and the Vps26-myc mutants indicated in the figure (left column shows Alexa 488 green channel), as well as the colocalization of these constructs with SNX1 (middle column shows Alexa 546, red channel), was examined in fixed/permeabilized cells by indirect immunofluorescence and confocal microscopy. The right-hand column shows merged images; yellow indicates co-localization.