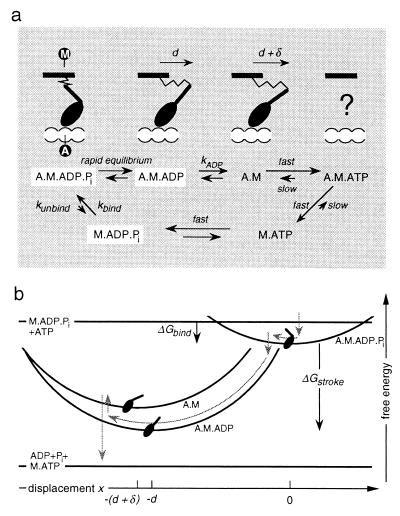
Figure 1.
(a) Biochemical cycle of myosin, showing the conformation of the head domain that corresponds to each chemical state. M labels the point of attachment of the head to the thick filament and A labels the binding site on the thin filament. Because of the fast reactions, only the three states boxed in white are populated to a significant degree. Note that there are variations of this reaction scheme that do not substantially change the model; e.g., the binding rate could be limited by the rate at which M⋅ADP⋅Pi is created by hydrolysis of M⋅ATP. (b) Because of the linear elastic element in the myosin neck, the free energy of attached states varies quadratically with the displacement x of A relative to M (the elastic strain of A·M·ADP⋅Pi is taken to be zero at x = 0). The gray arrows indicate the typical reaction pathway of a myosin molecule when the thin filament is propelled by an ensemble of motor proteins.