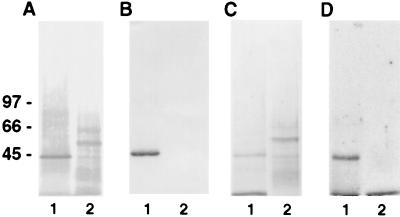
Figure 3.
Affinity purification of plasminogen and angiostatin binding sites. Plasma membranes were prepared as described in Materials and Methods. SDS/PAGE containing membrane proteins then were analyzed by Western blotting. Membranes were incubated in 10 mM Tris⋅HCl, 0.15 M NaCl, 0.05% Nonidet P-40, pH 7.5 containing (A) streptavidin-alkaline phosphatase conjugate antibody or (B) anti-annexin II antibody and developed by using 5-bromo-4-chloroindol-3-yl-phosphate nitro blue tetrazolium. Membrane stained with Coomassie brilliant blue (C), showing affinity-purified membrane proteins. Membrane incubated with 125I-labeled plasminogen (D), showing binding to the plasminogen-purified membrane and not the angiostatin. Lane 1 represents protein eluted from the plasminogen-Sepharose column. Lane 2 represents protein eluted from the angiostatin-Sepharose column. The relative molecular masses of α-ATP synthase and β-ATP synthase are ≈55 and ≈50 kDa, respectively.