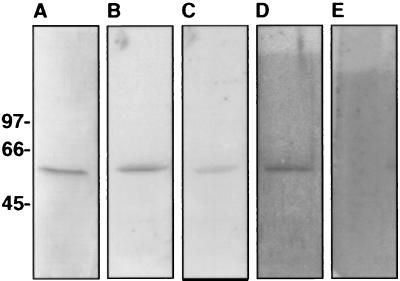
Figure 7.
Angiostatin binding to the recombinant α-subunit of human ATP synthase. The α-subunit of human ATP synthase was cloned and expressed in E. coli and purified by using Qiagen’s nickel-Sepharose protein purification system before dialyzing in PBS, pH 7.0. Recombinant protein was electrophoresed on 5–15% SDS/PAGE, electroblotted onto Immobilon membrane, and incubated 18 h in 10 mM Tris⋅HCl/0.15 M NaCl/0.05% Nonidet P-40, pH 7.5 (TSN buffer) containing 125I-angiostatin. For competition studies unlabeled ligand was added 4 h before radiolabeled ligand. Blots were washed in TSN buffer containing 0.05% Tween80 and bound radioactivity was quantified on a Molecular Dynamics PhosphorImager. (A) Coomassie stain of Immobilon membrane containing the α-subunit of human ATP synthase. (B) Binding of 0.5 μM 125I-labeled angiostatin. (C) Binding of 0.5 μM 125I-labeled angiostatin in the presence of a 250-fold molar excess of unlabeled angiostatin. Binding of angiostatin is inhibited by ≈56%. (D) Binding of 0.5 μM 125I-labeled angiostatin in the presence of a 2,500-fold molar excess of unlabeled plasminogen. Binding of angiostatin is not inhibited. (E) Binding of 0.5 μM 125I-labeled plasminogen to the α-subunit of human ATP synthase. Plasminogen did not bind to the recombinant α-subunit of ATP synthase; however, it did bind the annexin II control (as shown in Fig. 3).