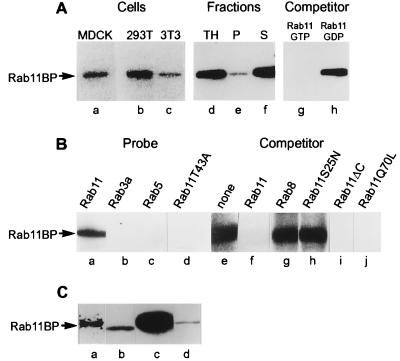
Figure 1.
Identification and cDNA cloning of a 130-kDa protein (rab11BP) that specifically interacts with rab11-GTP in a filter-overlay assay. (A) Lanes a–c, presence of rab11BP in MDCK (a), 293T (b), and 3T3 (c) cells. Cell lysates (100 μg of protein) were analyzed by the filter-overlay assay using [α-32P]GTP-rab11 as a probe. Lanes d–f, though primarily cytosolic (f), a fraction of rab11BP is membrane-associated (e). A total MDCK cell lysate (TH, lane d) and aliquots of sedimentable (P, lane e) and soluble subfractions (S, lane f) were analyzed by the blotting assay for the presence of rab11BP. Lanes g and h, only the active GTP-containing form of rab11 binds to rab11BP. Filter-overlay assays with MDCK cell lysates and [α-32P]GTP-rab11 as probe were carried out in the presence of a 30-fold excess of rab11 charged with nonradioactive GTP (g) or GDP (h). (B) Specificity of the rab11–rab11BP interaction. When used as probes charged with [α-32P]GTP, rab 3a (b), rab5 (c), and a rab11 effector domain mutant (rab11T43A) (d) fail to bind to rab11BP. Aliquots (100 μg protein) of a rat brain cytosolic subfraction obtained by ammonium sulfate precipitation (25–45% saturation) were analyzed by the blotting assay with the indicated [α-32P]GTP-charged probes. Lanes e–h, when used as competitors in the blotting assay with the brain cytosolic subfraction, rab8 and the dominant negative rab11 mutant (rab11S25N) do not diminish binding of [α-32P]GTP-rab11 to rab11BP. The various competitors were incubated for charging with cold GTP and used in the blotting assay at a 30-fold excess over the concentration of the labeled [32P]GTP-rab11 probe. (C) The cloned cDNA encodes a protein that comigrates with rab11BP and binds rab11-GTP. Lane a, an immunoprecipitate obtained from a partially purified brain cytosolic extract with an antibody to a peptide (residues 88–103) within the amino acid sequence derived from the cloned cDNA was used for the filter-overlay assay with [α-32P]GTP-rab11 as a probe. The protein in the immunoprecipitate that bound the probe (a) had the same electrophoretic mobility as rab11BP (d) in the original bovine brain protein fraction. Lanes b and c, aliquots of lysates of 293T cells transfected with the pcDNA vector alone (b) or the vector containing the cloned cDNA (c) were analyzed by the filter-overlay assay. The amount of extract analyzed in lane b, to reveal the endogenous human rab11BP, was 30 times greater than that from the transfected cells used in lane c.