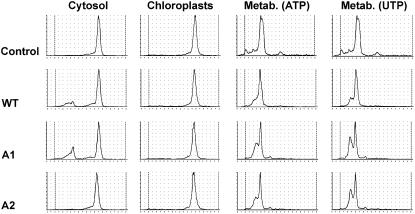
Figure 10.
Biochemical characterization of the Arabidopsis knockout mutants in At-xk-1 and At-xk-2 alleles. XK activities were measured in cytosolic fractions and chloroplastidic fractions. Protein extracts from wild-type Col-0 plants (WT), Atxk-1∷T-DNA (A1), and Atxk-2∷T-DNA (A2) knockout mutants were incubated in the presence of d-[U-14C]Xyl and Xyl isomerase. The protein extracts were incubated for 4 h in the presence of Tris-HCl 50 mm, MgCl2 20 mm, KF 10 mm, NTP 20 mm, and 3.05 mm d-[U-14C]Xyl. After incubation, an aliquot (2 μL) of the reaction mixture was directly spotted on a silica plate, and the product DXP (or XP) was separated from the substrate DX (or X) by TLC as described in previous legends and analyzed with a radioactivity TLC scanner (Berthold). The catalytical capacity of this protein extract was checked by apparent metabolization (Metab.) of XP in the presence of 6 mm ATP or UTP.