Abstract
Epithelial cells act as an interface between human mucosal surfaces and the surrounding environment. As a result, they are responsible for the initiation of local immune responses, which may be crucial for prevention of invasive infection. Here we show that epithelial cells detect the presence of bacterial pore-forming toxins – including pneumolysin from Streptococcus pneumoniae, α-hemolysin from Staphylococcus aureus, streptolysin O from Streptococcus pyogenes, and anthrolysin O from Bacillus anthracis – at nanomolar concentrations, far below those required to cause cytolysis. Phosphorylation of p38 MAPK appears to be a conserved response of epithelial cells to subcytolytic concentrations of bacterial PFT, and this activity is inhibited by the addition of high molecular weight osmolytes to the extracellular medium. By sensing osmotic stress caused by insertion of a sublethal number of pores into their membranes, epithelial cells may act as an early warning system to commence an immune response while the local density of toxin-producing bacteria remains low. Osmosensing may thus represent a novel innate immune response to a common bacterial virulence strategy.
Elaboration of pore-forming toxins (PFT) is a common theme in bacterial pathogenesis. Many distantly related species produce protein toxins capable of puncturing eukaryotic membranes, suggesting that this strategy may be an example of convergent evolution. However, the PFT themselves are a heterogeneous group, made up of several distinct structural classes. Typically PFT are secreted as monomers, which insert into host cell membranes, form homooligomers, and finally lead to disruption of membrane integrity (1). The consequences of pore formation for the host cell may be dire, leading to cell death by rupture or to the induction of apoptosis (2). This cytolysis may, in turn, confer a survival advantage to the bacterium, allowing it to better adhere to damaged cells, to enter previously inaccessible sites, or to escape immune responses. In many cases, most notably the major gram-positive pathogens Staphylococcus aureus, Streptococcus pneumoniae and Streptococcus pyogenes, PFT are essential for full virulence of the organism, though their precise mechanisms of action in vivo are not well understood (3–5).
Here we show that respiratory epithelial cells, the initial point of contact between many common bacterial pathogens and their human hosts, respond to sublytic, nanomolar concentrations of distinct classes of bacterial PFT through phosphorylation of the cytoplasmic protein p38 mitogen-activated protein kinase (MAPK). Activation of p38 MAPK during bacterial infection has been shown to be crucial to local production of cytokines such as the chemokine interleukin-8 (6) and to the development of an effective immune response in vivo (7). In this regard, we have previously observed that S. pneumoniae that lack the PFT pneumolysin induce lower chemokine responses associated with diminished neutrophil influx and delayed clearance in a murine model of mucosal colonization (8). Our findings are also consistent with the observation that survival of C. elegans challenged with a PFT appears to be critically dependent on its ability to activate members of the MAPK family (9). We demonstrate that toxin-induced p38 MAPK activation requires pore formation and is inhibited by relief of osmotic stress. This finding demonstrates a role for the epithelium in detection of bacterial PFT at sublytic concentrations and has implications for understanding the early stages of immune responses to bacterial products during colonization or disease.
METHODS
Bacterial strains and products
S. pneumoniae strains D39 (10) and its Ply-deficient derivative D39ply (3), kindly provided by D. Briles, were grown as described (6). S. aureus RN6390 and its α-hemolysin deficient derivative RN6390hla (A. Cheung) were grown in tryptic soy broth. Purified Ply and toxoid PdB (Ply-W433F) were the gift of J. Paton. Purified recombinant anthrolysin O (ALO) (11) was the gift of Richard Rest. Other reagents were from Sigma unless otherwise noted.
Epithelial cell lines and culture conditions
A549 (CCL-185), Detroit 562 (CCL-138), and human embryonic kidney 293 (HEK; CRL-1573) cells were grown in DMEM (Invitrogen) supplemented with 1 mM sodium pyruvate, 10% fetal bovine serum (HyClone), 100 U/ml penicillin and 100 mg/ml streptomycin.
Bacterial stimulation of epithelial cells and p38 MAPK Western blot
A549 or HEK or Detroit 562 cells grown to confluence in 6-well plates were weaned from serum and antibiotics overnight. Bacteria were grown in liquid culture to mid-log phase (OD620=0.5), collected by centrifugation, washed in PBS, and resuspended in DMEM without serum or antibiotics. Serial dilutions were plated to confirm bacterial density. 1×107 cfu/ml of S. pneumoniae or S. aureus was added to the epithelial monolayer (M.O.I. = ~10). In some experiments, PBS-washed bacteria were sonicated prior to use. Also, where indicated, bacterial toxins or purified TNF-α, diluted to the indicated concentrations in DMEM, were added to the cells in lieu of bacteria. Plates were spun at 150×g for 5 min to apply bacteria to epithelial cells and incubated at 37°C, 5% CO2 for the indicated incubation periods. After washing with sterile PBS, cells were lysed in 1% Triton X-100 with protease and phosphatase inhibitors and aliquots separated on a 10% polyacrylamide gel. Proteins were transferred to PVDF membranes and probed using the PhosphoPlus p38 MAPK Antibody Kit (Cell Signaling). Duplicate samples were run on separate gels for detection of total and phosphorylated p38 MAPK. Blots were not stripped and reprobed, though we repeated a subset of experiments using the technique of probing a membrane for phospho-p38 MAPK, stripping it, and reprobing for total p38 MAPK. In no case did this alter our observed results. In some experiments, A549 cells were loaded with BAPTA/AM (Invitrogen; 10 μM) for 45 min at 37°C, washed twice with PBS, and incubated in fresh DMEM for 45 min prior to stimulation. In experiments using dextran or cellulose (20 micron average particle size) and purified PFT, these reagents were added simultaneously. Because HEK cells adhere poorly if weaned from serum, unweaned cells were used in experiments with this cell line. Because A549 cells detach if incubated at 37°C in the presence of EGTA, experiments using EGTA were carried out at room temperature.
In all cases, epithelial cell viability exceeded 95%. As an independent marker for cell damage or death, release of lactate dehydrogenase into supernatants of cells exposed to toxins for 45 min at 37°C was assessed using a commercial kit (Roche). Positive control lysis was with 1% Triton X-100 in PBS.
Interleukin-8 ELISA
A549 cells were grown to confluence in 96-well plates and incubated in serum-free DMEM overnight. Quadruplicate wells were treated with Ply or PdB diluted to indicated concentrations in serum-free DMEM. Where indicated, pretreatment of cells with 20 mM SB203580 (Calbiochem) was for 30 min at 37°C and SB203580 was present during incubation of A549 cells with toxin or toxoid preparations. After 18 hrs, supernatants were removed and the concentration of interleukin (IL)-8 determined using a commercially available ELISA (Pharmingen OptEIA). Conditions were compared by one way ANOVA with Tukey test for multiple comparisons (Prism; GraphPad Software).
Hemolytic Assay
Purified Ply or PdB was diluted in PBS. 80 μl of diluted toxin was added to 160 μl lysis buffer (PBS + 0.1% BSA, 10 mM DTT) and 80 μl PBS-washed horse erythrocytes (2%) in 96-well V-bottomed plates. The plate was incubated at 37°C for 30 min, centrifuged at 150 × g for 10 min, and supernatants collected. Optical density at 415 nm was recorded in a plate reader (BioRad). Triplicate wells at each concentration were assayed, and each experiment was repeated at least three times. In some experiments, 5 mM EGTA was added to the buffer prior to the addition of toxin or erythrocytes. MgCl2 or CaCl2 in PBS was added to the buffer along with EGTA to give indicated concentrations.
RESULTS AND DISCUSSION
PFT are necessary and sufficient for activation of p38 MAPK
Prior studies have demonstrated activation of p38 MAPK in the setting of pneumococcal pneumonia as an essential component of host control of bacterial replication in the lung (7). In addition, activation of p38 MAPK in human cells in vitro by S. pneumoniae has been described (12,13). Stringaris and colleagues reported activation of p38 MAPK and calcium-dependent cell death in neuroblastoma cells treated with purified Ply and speculated on its relevance to the pathogenesis of pneumococcal meningitis (14). Likewise, S. aureus activation of host p38 MAPK has been recognized but not attributed to the pore-forming nature of its α-hemolysin (15). S. pyogenes has been reported to activate osteoblasts and keratinocytes via p38 MAPK, but the importance of SLO in these systems was not addressed (16,17).
Because it is required for synergistic enhancement of epithelial cytokine production during polymicrobial infection (6), we hypothesized that pneumolysin (Ply), the single protein toxin of S. pneumoniae, might be sufficient to induce p38 MAPK phosphorylation in respiratory epithelial cells. Treatment of A549 respiratory epithelial cells with S. pneumoniae D39, but not its isogenic Ply-deficient mutant D39ply led to phosphorylation of p38 MAPK as determined by Western blotting (Fig. 1A). In order to assess whether this was a property specific to S. pneumoniae and its toxin, we performed a similar experiment using S. aureus, another major gram-positive pathogen. Importantly, while S. aureus does produce pore-forming toxins, of which α-hemolysin is the most abundant (7), these are structurally unrelated to Ply and form considerably smaller pores in eukaryotic cells. Despite these differences, we found that wild-type S. aureus RN6390 activated epithelial p38 MAPK in a similar manner to S. pneumoniae. Correspondingly, the isogenic α-hemolysin deficient mutant RN6390hla was attenuated in its ability to activate epithelial p38 (Fig. 1B).
Figure 1.
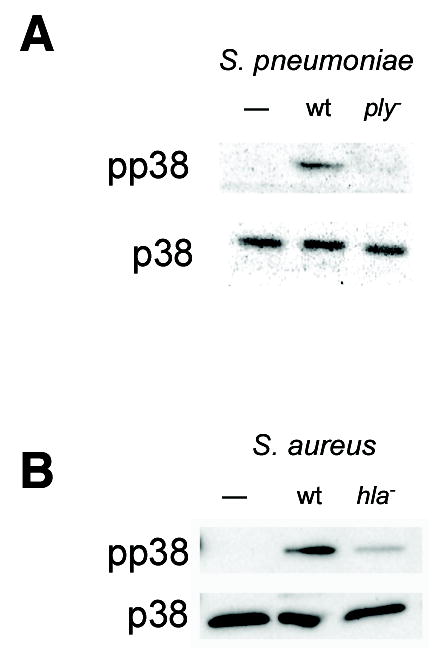
Pore-forming toxins are essential for bacterial activation of epithelial p38 MAPK. Confluent monolayers of A549 cells were stimulated for 30 min with 1×107 cfu/ml of S. pneumoniae D39 or its isogenic Ply-deficient mutant, D39ply (A) or with S. aureus RN6390 or its isogenic α-hemolysin mutant, RN6390hla (B). In both cases, the wild-type, but not the toxin-deficient mutant, led to phosphorylation of p38 MAPK as assessed by Western blotting with antibodies specific for total p38 MAPK (p38) and phosphorylated p38 (pp38).
In order to confirm that PFT were sufficient as well as necessary for activation of epithelial p38 MAPK in response to gram-positive bacteria, we stimulated epithelial cells with purified S. aureus α-hemolysin, Ply, or other members of the cholesterol-dependent cytolysin family, streptolysin O (SLO) and anthrolysin O (ALO) (Fig. 2). In each case, purified PFT was sufficient to cause p38 phosphorylation in epithelial cells and required either high ng/ml or low μg/ml quantities (corresponding to nanomolar concentrations). These in vitro results correlated with and may account for our prior observations that Ply-expressing but not non-expressing S. pneumoniae induce mucosal production of macrophage inflammatory protein-2 (a murine homolog of interleukin-8) and subsequent recruitment of neutrophils during murine nasopharyngeal colonization (8). At the toxin concentrations used, epithelial cell viability was unaffected and exceeded 95% as assessed by trypan blue exclusion. In addition, LDH release from A549 cells treated with toxins at these concentrations was less than 5% of the positive control lysis in all cases (data not shown). At a ten-fold higher concentration of Ply (1 μg/ml) we noted release of >65% of total cellular LDH. Thus, we found that subcytolytic concentrations of each toxin led to increased p38 MAPK phosphorylation.
Figure 2.
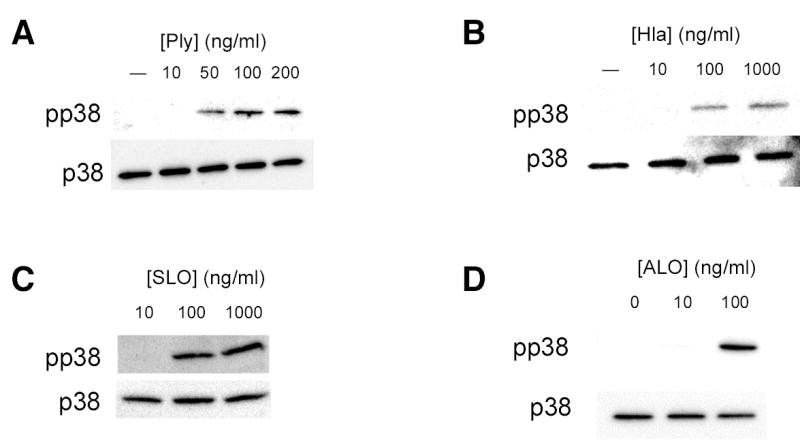
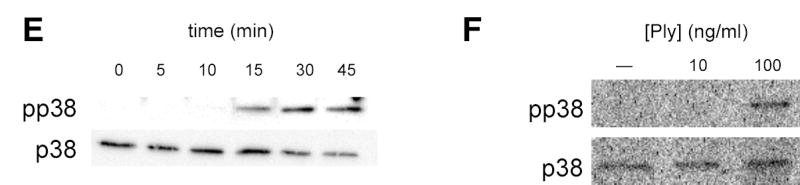
Purified bacterial pore-forming toxins activate epithelial p38 MAPK. Confluent monolayers of A549 cells were treated with the indicated concentrations of purified pneumolysin (Ply) (A), S. aureus α-hemolysin (hla) (B), streptolysin O (SLO) (C), or anthrolysin O (ALO) (D) for 30 min or with 100 ng/ml Ply for the indicated times (E). Total and phosphorylated p38 MAPK were assessed by Western blotting. Similar responses were noted in Detroit 562 nasopharyngeal epithelial cells stimulated with Ply (F).
Phosphorylation of p38 MAPK was detectable within 15 min after exposure of cells to toxin and reached its maximal level by 30 min (Fig. 2E). Likewise, we noted p38 MAPK phosphorylation in response to Ply in an unrelated cell line, Detroit 562 nasopharyngeal cells, suggesting that these effects are not specific to A549 cells (Fig. 2F). We attempted to confirm p38 MAPK phosphorylation in response to PFT in primary murine epithelial cells but were hampered by endogenous phosphorylation of p38 MAPK, making differences difficult to assess (data not shown).
Toxin-mediated pore formation activates p38 MAPK
It has been proposed that some PFT have effects on eukaryotic cells that are independent of pore-formation. Specifically, Ply and other members of the cholesterol-dependent cytolysins are putative ligands for the host pattern-recognition molecule toll-like receptor (TLR)-4 independent of their pore-forming activities (18–20). Non-hemolytic Ply toxoids are equally efficient compared to native Ply as TLR4 agonists (18). Because S. aureus and S. pneumoniae produce PFT that are not structurally related yet both induce epithelial p38 MAPK phosphorylation, we hypothesized that pore-formation, rather than recognition of specific toxin motifs, might be an essential step in the p38 MAPK response to toxins. We used the Ply toxoid PdB, which has a point mutation in the region that is essential for pore formation, to distinguish the pore-forming properties of Ply from its potential action on receptors such as TLR4. PdB inserts into cholesterol-containing membranes and oligomerizes but does not form functional pores (21). In order to confirm the non-cytolytic quality of PdB, we used an assay of horse erythrocyte hemolysis (Fig. 3A). Measurement of hemoglobin release from horse erythrocytes is a convenient measure of pore-formation, though it takes place at substantially lower doses than those relevant to epithelial cell stimulation.
Figure 3.
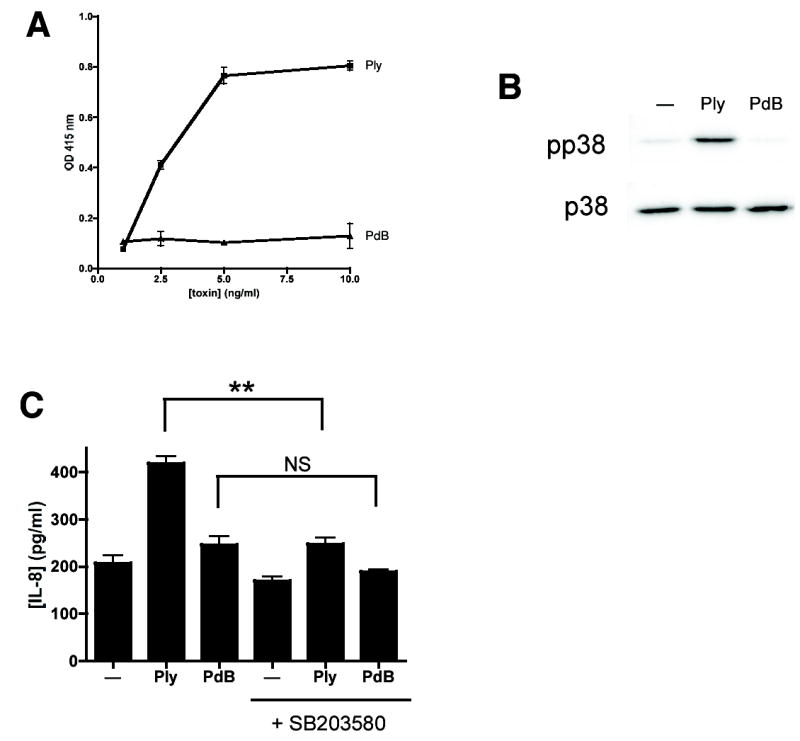
Pore formation is essential for Ply-mediated p38 MAPK activation and interleukin-8 release from epithelial cells. Horse erythrocytes were incubated with the indicated concentrations of purified Ply for 30 min. Optical density at 415 nm was used to assess hemoglobin release from lysed cells (A). Native Ply (100 ng/ml), but not its non-cytolytic toxoid PdB (100 ng/ml) or treatment with media alone, activates p38 MAPK in A549 cells (B). Treatment of A549 cells with Ply (100 ng/ml) but not PdB (100 ng/ml) leads to interleukin-8 release as measured by ELISA. Pretreatment of cells with SB203580, an inhibitor of p38 MAPK, abolishes this effect (C). **, P < 0.001; NS, not significant (P > 0.05).
Treatment of A549 cells with purified Ply, but not with equivalent concentrations of PdB led to phosphorylation of p38 MAPK (Fig. 3B). Similar results were obtained using HEK cells, which lack TLR2 and TLR4 (22), suggesting that the mechanism of p38 activation is independent of these receptors (data not shown). This finding, as well as the observation that structurally unrelated PFT activate p38 MAPK, suggests that pore-formation, rather than any effect of Ply or PdB on TLR4-mediated signaling, is the mechanism of toxin-induced activation of this host cell pathway.
Epithelial IL-8 response to PFT requires p38 MAPK activity
Consistent with these findings, treatment of epithelial cells with Ply but not PdB led to release of interleukin-8, a chemokine that functions as a potent neutrophil attractant. The chemical inhibitor of p38 MAPK activity, SB203580, was sufficient to reduce or abolish this response (Fig. 3C). Activation of extracellular signal related kinase (ERK 1/2) and c-Jun N-terminal kinase (JNK) were also detected in response to PFT (data not shown). However, we focused our investigations on the mechanism of the p38 MAPK response because SB203580 inhibited epithelial IL-8 release in response to microbial products (Fig. 3C and (6)).
Divalent cations enhance toxin-mediated pore formation
Studies of the effects of purified Ply on neutrophils have suggested a role for extracellular calcium in the initiation of host cell responses. Typically, chelation of divalent cations using EGTA has been associated with inhibition of Ply-mediated effects. The mechanism of this effect has been hypothesized to involve entry of Ca ions through Ply pores (23–26). This is consistent with studies of PFT from E. coli, showing that the insertion of pores into host cell membranes can induce calcium oscillations and transient currents that may activate host cell signaling pathways (27). We further investigated the role of divalent cations in Ply-induced host cell signaling.
Chelation of extracellular divalent cations with EGTA eliminated the ability of Ply to induce p38 MAPK phosphorylation in A549 cells (Fig. 4A). However, this finding did not clarify whether the presence of divalent cations was important for pore formation or was a downstream effect essential for p38 MAPK activation (such as entry of cations through Ply pores). In order to explore this further, we used the cell-permeant acetoxymethyl ester of BAPTA, a related cation chelator. Consistent with the hypothesis that pore formation, rather than cation flux, is important for activation of p38 MAPK by bacterial PFT, chelation of intracellular divalent cations with BAPTA/AM had no effect on Ply-mediated p38 MAPK activation (Fig. 4B). Concentrations of BAPTA/AM used in these experiments have been previously shown to efficiently inhibit Ca2+-dependent pathways in epithelial cells (15). In our system, BAPTA/AM did enhance phosphorylation of ERK in A549 cells treated with thapsigargin (1 μM, 15 min) (data not shown) as previously described in hepatic epithelial cells (28).
Figure 4.
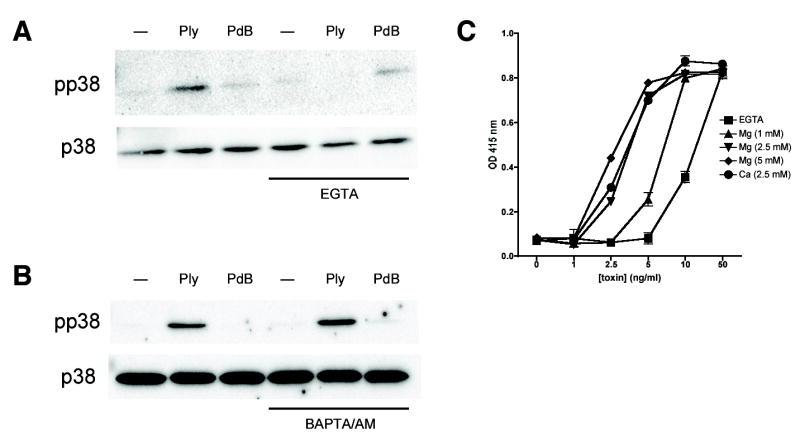
Extracellular divalent cations are required for p38 MAPK activation and pore formation by Ply. Pretreatment of A549 cells with the extracellular divalent cation chelator EGTA (5 mM) inhibits p38 MAPK phosphorylation in response to Ply (100 ng/ml, 30 min) (A). In contrast, the cell-permeant chelator BAPTA/AM (10 μM) has no effect on toxin-mediated p38 MAPK phosphorylation (B). Horse erythrocyte lysis by Ply at the concentrations indicated is inhibited in the presence of EGTA and is restored by the addition of MgCl2 or CaCl2 to the extracellular medium (C).
EGTA chelation of extracellular divalent cations also increased the concentration of Ply needed to achieve lysis of horse erythrocytes relative to conditions in which divalent cations were present (Fig. 4C), suggesting that these cations may be a previously unrecognized requirement for efficient formation of its pores. In order to confirm the dependence of this effect on the chelation capacity of EGTA, we added Mg or Ca salts in the presence of EGTA. Addition of either MgCl2 or CaCl2 increased the efficiency of Ply-mediated hemolysis. This is consistent with findings related to another, unrelated, PFT, α-latrotoxin, which requires divalent cations for efficient pore formation (29). Prior studies of Ply pores demonstrated that small- and medium-sized Ply channels were gated by divalent cations whereas large ones were insensitive to the effects of these cations (21,30). Our results suggest that it may be the larger, cation-insensitive pores that are responsible for the majority of cellular effects, including erythrocyte lysis and epithelial p38 MAPK activation.
Epithelial cells sense PFT via osmotic stress
Because p38 MAPK phosphorylation is involved in osmotic stress sensation (31), we hypothesized that activation of p38 MAPK by PFT might be a consequence of cellular processes normally used to sense membrane integrity. If that were the case, relief of osmotic stress caused by sublethal disruption of membrane integrity by PFT ought to ameliorate p38 activation. We used high molecular weight dextran (M.W. ~167,000) larger than the cutoff size previously shown to enter through pores created by a related toxin, SLO, in prior work (32). Dextrans of this size are sufficient to inhibit toxin-induced hemolysis through osmotic protection (21). Addition of high molecular weight dextran to the extracellular medium efficiently inhibited the p38 response of cells to Ply (Fig. 5A) or to S. aureus α-hemolysin (Fig. 5B), but not to TNF-α treatment (Fig. 5B), consistent with the hypothesis that osmosensing plays an important role in this epithelial response to PFT. The absence of an effect on TNF-mediated p38 MAPK phosphorylation suggests that the effect of dextran is specific to signaling initiated by PFT. Likewise, a suspension of cellulose particles of 20 μm diameter inhibited the response to PFT (Fig. 5C). In contrast, osmolytes of smaller sizes such as sucrose (50–150 mM), which would be expected to enter through the Ply pore, did not inhibit p38 MAPK phosphorylation in response to PFT (data not shown). Inhibition of p38 MAPK phosphorylation by dextran or cellulose makes it unlikely that impurities in any of our toxin preparations are responsible for the effects observed, as does the difference in response between Ply and PdB, both of which were purified from E. coli under identical conditions but which exhibited strikingly different activities with respect to phosphorylation of p38 MAPK.
Figure 5.
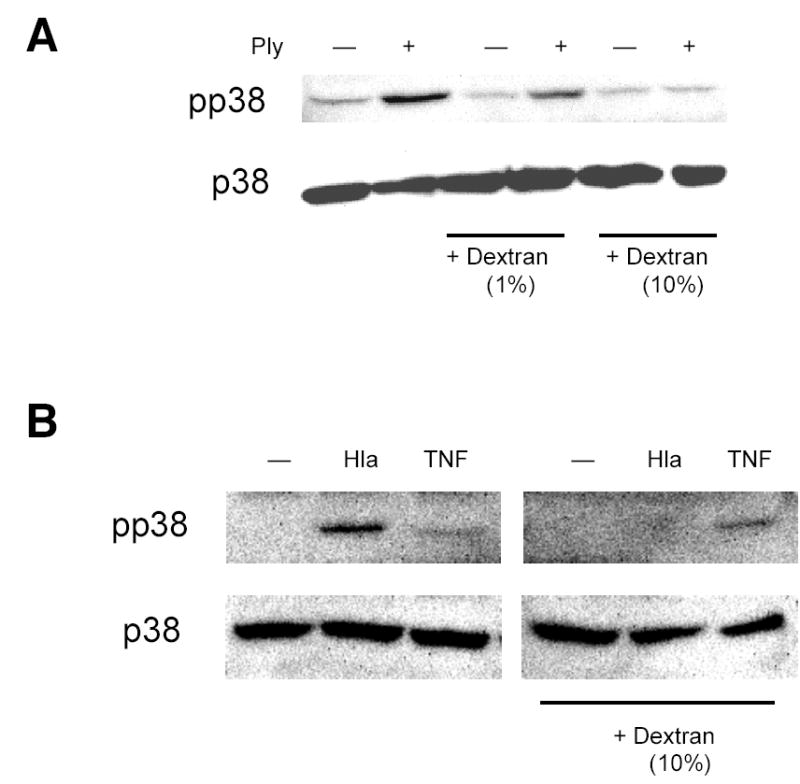
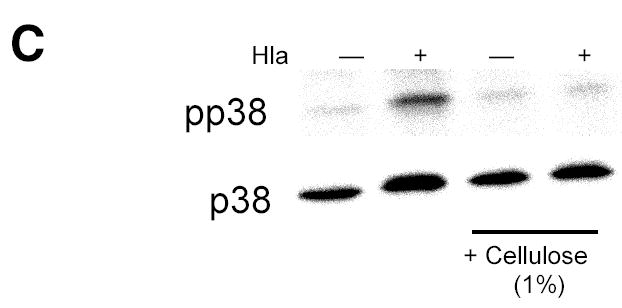
Sensation of osmotic stress mediates toxin-induced p38 MAPK phosphorylation. Addition of high molecular weight dextran (M.W. 167,000) to the extracellular medium inhibits p38 MAPK phosphorylation following treatment with Ply (100 ng/ml, 30 min) (A) or hla (100 ng/ml, 30 min) (B) but not with TNF-α (10 ng/ml, 5 min) (B). Cellulose suspension (20 μm particle size, 1%) inhibits p38 MAPK phosphorylation in A549 cells exposed to hla (100 ng/ml, 30 min).
Our finding of crosstalk between mechanisms of pathogen recognition and epithelial osmosensing is a novel observation. Additionally, detection of sublytic, nanomolar concentrations of bacterial PFT may provide a mechanism for epithelial cells to initiate proinflammatory responses early in infection, when the bacterial density is still low. These observations have implications for our understanding of immune responses to pathogens at mucosal surfaces. Pathways that sense cell stress may represent a novel arm of the mucosal innate immune response.
Acknowledgments
The authors thank Howard Goldfine for careful reading of the manuscript. We thank Richard Rest, Elise Mosser, James Paton, Ambrose Cheung, and David Briles for reagents. This study was supported by NIH (Grants AI054647, AI044231, AI038446 to J.N.W.; AI065450 to A.J.R.) and the PIDS-St. Jude Fellowship Program (A.J.R.)
References
- 1.Burns DL. ASM Press; Washington, D.C: 2003. Bacterial protein toxins. [Google Scholar]
- 2.Braun JS, Sublett JE, Freyer D, Mitchell TJ, Cleveland JL, Tuomanen EI, Weber JR. J Clin Invest. 2002;109(1):19–27. doi: 10.1172/JCI12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berry AM, Yother J, Briles DE, Hansman D, Paton JC. Infect Immun. 1989;57(7):2037–2042. doi: 10.1128/iai.57.7.2037-2042.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kernodle DS, Voladri RK, Menzies BE, Hager CC, Edwards KM. Infect Immun. 1997;65(1):179–184. doi: 10.1128/iai.65.1.179-184.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Limbago B, Penumalli V, Weinrick B, Scott JR. Infect Immun. 2000;68(11):6384–6390. doi: 10.1128/iai.68.11.6384-6390.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ratner AJ, Lysenko ES, Paul MN, Weiser JN. Proc Natl Acad Sci U S A. 2005;102(9):3429–3434. doi: 10.1073/pnas.0500599102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van den Blink B, Juffermans NP, ten Hove T, Schultz MJ, van Deventer SJ, van der Poll T, Peppelenbosch MP. J Immunol. 2001;166(1):582–587. doi: 10.4049/jimmunol.166.1.582. [DOI] [PubMed] [Google Scholar]
- 8.van Rossum AMC, Lysenko ES, Weiser JN. Infect Immun. 2005;73(11):7718–7726. doi: 10.1128/IAI.73.11.7718-7726.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huffman DL, Abrami L, Sasik R, Corbeil J, van der Goot FG, Aroian RV. Proc Natl Acad Sci U S A. 2004;101(30):10995–11000. doi: 10.1073/pnas.0404073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avery OT, MacLeod CM, McCarty M. J Exp Med. 1944;79:137–158. doi: 10.1084/jem.79.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shannon JG, Ross CL, Koehler TM, Rest RF. Infect Immun. 2003;71(6):3183–3189. doi: 10.1128/IAI.71.6.3183-3189.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monier RM, Orman KL, Meals EA, English BK. J Infect Dis. 2002;185(7):921–926. doi: 10.1086/339483. [DOI] [PubMed] [Google Scholar]
- 13.Schmeck B, Zahlten J, Moog K, van Laak V, Huber S, Hocke AC, Opitz B, Hoffmann E, Kracht M, Zerrahn J, Hammerschmidt S, Rosseau S, Suttorp N, Hippenstiel S. J Biol Chem. 2004;279(51):53241–53247. doi: 10.1074/jbc.M313702200. [DOI] [PubMed] [Google Scholar]
- 14.Stringaris AK, Geisenhainer J, Bergmann F, Balshusemann C, Lee U, Zysk G, Mitchell TJ, Keller BU, Kuhnt U, Gerber J, Spreer A, Bahr M, Michel U, Nau R. Neurobiol Dis. 2002;11(3):355–368. doi: 10.1006/nbdi.2002.0561. [DOI] [PubMed] [Google Scholar]
- 15.Ratner AJ, Bryan R, Weber A, Nguyen S, Barnes D, Pitt A, Gelber S, Cheung A, Prince A. J Biol Chem. 2001;276(22):19267–19275. doi: 10.1074/jbc.M007703200. [DOI] [PubMed] [Google Scholar]
- 16.Chung WO, Dale BA. Infect Immun. 2004;72(1):352–358. doi: 10.1128/IAI.72.1.352-358.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okahashi N, Sakurai A, Nakagawa I, Fujiwara T, Kawabata S, Amano A, Hamada S. Infect Immun. 2003;71(2):948–955. doi: 10.1128/IAI.71.2.948-955.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malley R, Henneke P, Morse SC, Cieslewicz MJ, Lipsitch M, Thompson CM, Kurt-Jones E, Paton JC, Wessels MR, Golenbock DT. Proc Natl Acad Sci U S A. 2003;100(4):1966–1971. doi: 10.1073/pnas.0435928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park JM, Ng VH, Maeda S, Rest RF, Karin M. J Exp Med. 2004;200(12):1647–1655. doi: 10.1084/jem.20041215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srivastava A, Henneke P, Visintin A, Morse SC, Martin V, Watkins C, Paton JC, Wessels MR, Golenbock DT, Malley R. Infect Immun. 2005;73(10):6479–6487. doi: 10.1128/IAI.73.10.6479-6487.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korchev YE, Bashford CL, Pederzolli C, Pasternak CA, Morgan PJ, Andrew PW, Mitchell TJ. Biochem J. 1998;329 (Pt 3):571–577. doi: 10.1042/bj3290571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nonnenmacher C, Dalpke A, Zimmermann S, Flores-De-Jacoby L, Mutters R, Heeg K. Infect Immun. 2003;71(2):850–856. doi: 10.1128/IAI.71.2.850-856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fickl H, Cockeran R, Steel HC, Feldman C, Cowan G, Mitchell TJ, Anderson R. Clin Exp Immunol. 2005;140(2):274–281. doi: 10.1111/j.1365-2249.2005.02757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cockeran R, Durandt C, Feldman C, Mitchell TJ, Anderson R. J Infect Dis. 2002;186(4):562–565. doi: 10.1086/341563. [DOI] [PubMed] [Google Scholar]
- 25.Cockeran R, Steel HC, Mitchell TJ, Feldman C, Anderson R. Infect Immun. 2001;69(5):3494–3496. doi: 10.1128/IAI.69.5.3494-3496.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cockeran R, Theron AJ, Steel HC, Matlola NM, Mitchell TJ, Feldman C, Anderson R. J Infect Dis. 2001;183(4):604–611. doi: 10.1086/318536. [DOI] [PubMed] [Google Scholar]
- 27.Uhlen P, Laestadius A, Jahnukainen T, Soderblom T, Backhed F, Celsi G, Brismar H, Normark S, Aperia A, Richter-Dahlfors A. Nature. 2000;405(6787):694–697. doi: 10.1038/35015091. [DOI] [PubMed] [Google Scholar]
- 28.Maloney JA, Tsygankova OM, Yang L, Li Q, Szot A, Baysal K, Williamson JR. Am J Physiol. 1999;276(1 Pt 1):C221–230. doi: 10.1152/ajpcell.1999.276.1.C221. [DOI] [PubMed] [Google Scholar]
- 29.Orlova EV, Rahman MA, Gowen B, Volynski KE, Ashton AC, Manser C, van Heel M, Ushkaryov YA. Nat Struct Biol. 2000;7(1):48–53. doi: 10.1038/71247. [DOI] [PubMed] [Google Scholar]
- 30.Korchev YE, Bashford CL, Pasternak CA. J Membr Biol. 1992;127(3):195–203. doi: 10.1007/BF00231507. [DOI] [PubMed] [Google Scholar]
- 31.Uhlik MT, Abell AN, Johnson NL, Sun W, Cuevas BD, Lobel-Rice KE, Horne EA, Dell’Acqua ML, Johnson GL. Nat Cell Biol. 2003;5(12):1104–1110. doi: 10.1038/ncb1071. [DOI] [PubMed] [Google Scholar]
- 32.Walev I, Hombach M, Bobkiewicz W, Fenske D, Bhakdi S, Husmann M. FASEB J. 2002;16(2):237–239. doi: 10.1096/fj.01-0572fje. [DOI] [PubMed] [Google Scholar]


