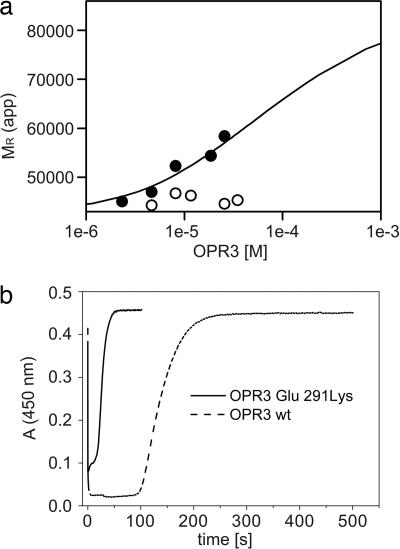
Fig. 4.
Biochemical analysis of the OPR3 dimer. (a) Sedimentation equilibrium of WT OPR3 (filled circles) and the Glu-291–Lys mutant (open circles) was analyzed at a protein concentration of 1–34 μM in the presence of 50 μM FMN. For WT OPR3, the apparent molecular masses were fitted to a monomer dimer equilibrium, yielding a dissociation constant (Kd) of 30 μM. (b) Enzyme-monitored turnover. WT OPR3 (solid line) and Glu-291–Lys OPR3 (dashed line) at 86 μM were mixed in the stopped-flow instrument with 1.4 mM NADPH and 1.5 mM trans-hex-2-enal (100 mM potassium phosphate, pH 7; 25°C). In both cases, rapid reduction by NADPH was observed (time = 0 s), followed by a steady-state phase. After exhaustion of NADPH, the enzyme returned to its oxidized state, as indicated by the recovery of absorbance at 450 nm.