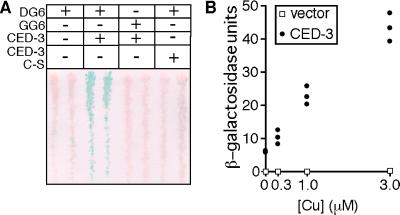
Figure 2.
Yeast expressing CLBDG6 act as reporters for CED-3 caspase activity. W303α yeast were transformed with pSH18–34, which carries a LexA-responsive lacZ transcriptional cassette (the LexA/β-gal reporter strain). These cells were transformed with pGALL expression plasmids carrying CLBDG6 (DG6) or CLBGG6 (GG6). These cells also carry a copper-inducible pCUP1 plasmid, which contains either wild-type CED-3 (CED-3), an inactive C to S mutant version of CED-3 (CED-3 C-S), or nothing. Duplicate colonies from each transformation were streaked onto gal/raf medium to induce GAL1-dependent expression of the caspase substrates and then were lifted onto complete media plates with 3 μM copper sulfate to induce caspase expression. After a 12-hr induction, an X-gal assay was performed on the filter. Only cells expressing CLBDG6 and wild-type CED-3 have significant β-gal activity (A). Cultures from three transformants carrying pSH18–34, pGAL-CLBDG6, and either the empty pCUP1 vector or pCUP1-CED-3 were grown to stationary phase, then were diluted into medium containing the indicated levels of copper sulfate and grown for a further 10 hr. o-nitrophenyl-β-d-galactoside assays were performed, and β-gal activity was determined. β-gal activity in the CED-3-expressing cells increased as a function of copper concentration (filled circles, CED-3). No β-gal activity was found in the cultures carrying only the empty pCUP1 vector (open boxes, vector) (B).