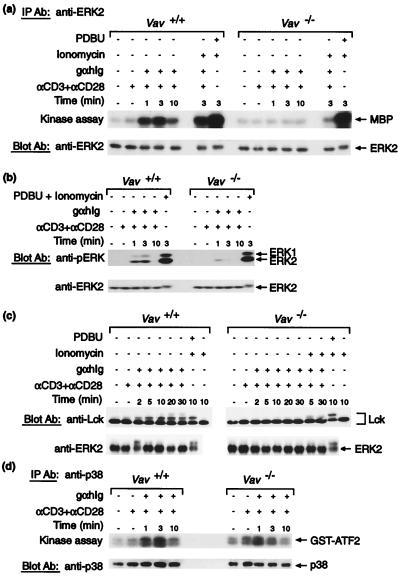
Figure 5.
Activation of MAPK pathways. CD4+ splenic T cells purified from Vav+/+ or Vav−/− mice were stimulated as described in Figs. 2 and 4. (a) ERK2 MAPK was immunoprecipitated from stimulated cells and used in an in vitro kinase assay with 32P-ATP to phosphorylate myelin basic protein (MBP). (Upper) An autoradiograph of a blot showing phosphorylation of myelin basic protein. In the lower panel the same blot has been probed with an anti-ERK2 antibody to control for loading. (b) Immunoblot of stimulated cell extracts probed with an anti-phosphoERK antibody and reprobed with an anti-ERK2 antibody to control for loading. Phosphorylation of the ERK1 and ERK2 kinases is indicative of their activation by the MEK kinase. (c, Upper) an immunoblot of stimulated cell extracts probed with an anti-Lck antibody. The shift in mobility of Lck to a slower migrating form of higher apparent molecular weight is caused by serine phosphorylation, which is likely to be downstream of ERK activation (26). Tyrosine phosphorylation of Lck does not cause this mobility shift. This shift is readily seen on 7–15% acrylamide gradient gels. (c, Lower) A blot of the same samples probed with an anti-ERK2 antibody showing the phosphorylation-induced mobility shift. In this experiment ionomycin was used at 500 ng/ml. These shifts are only seen upon extended electrophoresis; they were not seen in a and b as electrophoresis times were too short. (d) p38 MAPK was immunoprecipitated from stimulated cells and used in an in vitro kinase assay with 32P-ATP to phosphorylate a GST–ATF2 fusion protein. (Upper) An autoradiograph of the blot showing phosphorylation of GST–ATF2. In the lower panel the same blot has been probed with an anti-p38 antibody to control for loading.