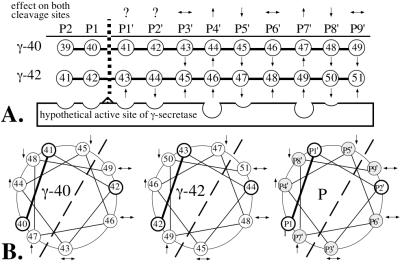
Figure 5.
Schematic representation of the amino acid positions (P) of A4CT relative to the cleavage site of γ-secretase. (A) Linear arrangement of the amino acids relative to the cleavage site after residue 40 (γ-40) or 42 (γ-42). The arrows (based on the data in Fig. 3) indicate whether the phenylalanine mutants of A4CT were cleaved at one site (γ-40 or γ-42) more (↑) or less (↓) efficiently than at the other site, compared with A4CT-wt. The scissile peptide bond, which is shown by a broken vertical line, is constituted by the residues in positions P1 and P1′. The arrows above the P positions show whether the mutations at the individual positions had the same effect (↑ or ↓) or the opposite effect (↔) on both cleavage sites. Question marks indicate that the effect of the mutation has been determined only for one cleavage site. (B) Helical wheel arrangement of amino acids 43 to 52 of A4CT with respect to the cleavage sites after residues 40 (γ-40) and 42 (γ-42). Helical arrangement P shows the amino acid positions in the transmembrane domain of A4CT in general. The scissile peptide bond (P1–P1′) is shown with a solid bold line. Those amino acid positions for which the effect of the phenylalanine mutations has been determined for both cleavage sites are shaded. The dashed line separates the helical wheel into two halves, one of which is supposed to interact directly with γ-secretase, whereas the other half is likely to face away from γ-secretase.