Figure 2.
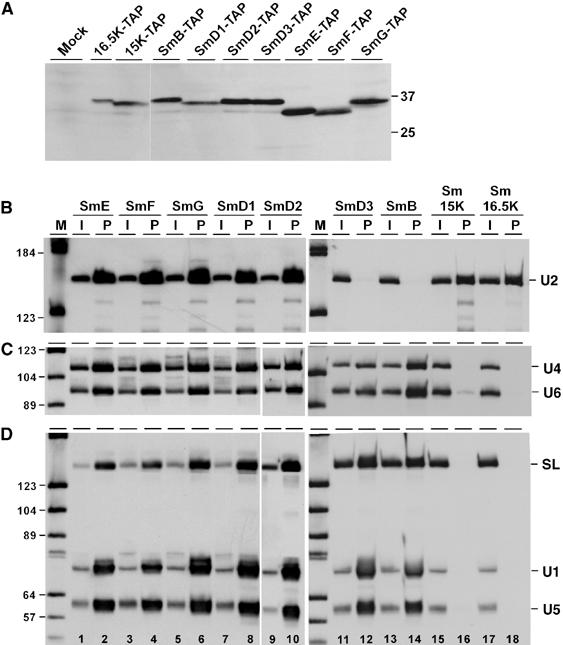
Both Sm15K and 16.5K proteins are U2-specific. (A) Stable expression of TAP-tagged Sm proteins. Lysates were prepared from the wild-type strain (lane mock) and all T. brucei cell lines stably expressing TAP-tagged Sm proteins (as indicated above the lanes). Protein was analyzed by SDS–polyacrylamide gel electrophoresis and Western blotting with peroxidase anti-peroxidase (PAP) soluble complex (Sigma). The positions of marker proteins are given on the right (sizes in kDa). (B, C, D) Extract was prepared from T. brucei cell lines, which stably express TAP-tagged versions of either of the seven canonical Sm proteins (SmE, SmF, SmG, SmD1, SmD2, SmD3, SmB), or of Sm15K or Sm16.5K proteins (as indicated above the lanes). TAP-tagged complexes were affinity-purified from each cell line, and copurifying RNAs were analyzed by Northern blotting, using a U2-specific probe (B), or mixed probes detecting U4 and U6 snRNAs (C), or SL RNA, U1, and U5 snRNAs (D). The snRNA positions are indicated on the right. The input in panel B represents 5% of the total material, in panels C and D 1% (see lanes I); all of the precipitated material is shown (see lanes P). Note that the probe mixture used for the SmD3, SmB, Sm15K, and Sm16.5K pull-downs contains relatively more SL probe than the probe mixture used for the other Sm polypeptides (e.g., compare input lanes 1 and 11, panel D). M, DIG marker V (Roche).
