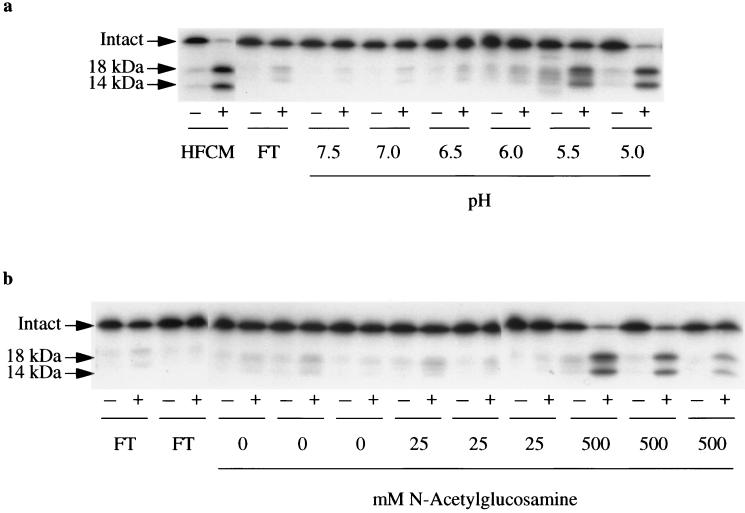
Figure 1.
Purification of the IGF-dependent IGFBP-4 protease from HFCM. (a) Metal-chelating affinity chromatography. Bound proteins were eluted with a descending stepwise pH gradient, and 50 μl aliquots of each of these fractions were dialyzed and assayed for IGFBP-4 protease activity. IGF-dependent IGFBP-4 protease activity is defined as the loss of intact 24-kDa [125I]IGFBP-4 and the appearance of 18- and 14-kDa radiolabeled fragments (denoted by arrows) in the presence (+), but not in the absence (-), of 5 nM IGF-II. HFCM, starting material; FT, flowthrough. (b) Wheat-germ agglutinin chromatography. Metal-chelating affinity chromatography fraction eluting at pH 5.0 was adjusted to pH 7.4 and immediately passed over a wheat-germ agglutinin column. Non- and weakly bound proteins came out in the flowthrough (FT) or were eluted with three washes of 0 mM and three washes of 25 mM N-acetylglucosamine. IGF-dependent IGFBP-4 protease activity was eluted with three washes of 500 mM N-acetylglucosamine.