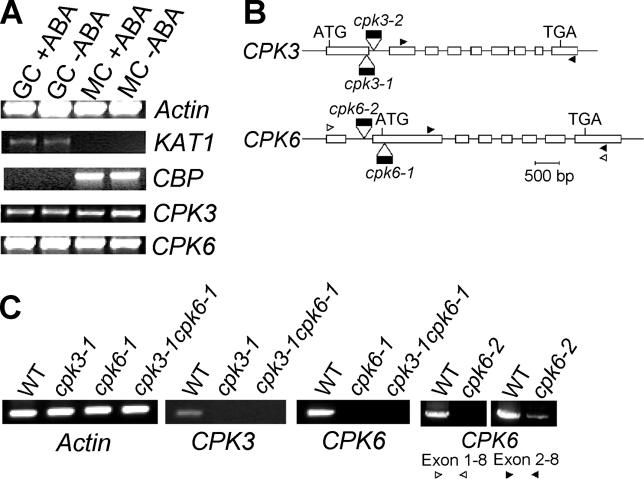
Figure 1. Guard Cell Expression of CPK3 and CPK6 CDPKs.
(A) Expression of CPK3 and CPK6 in guard cell (GC) and mesophyll cell (MC) protoplasts was examined by RT-PCR. Control amplifications of the guard cell-expressed KAT1 gene and the mesophyll-expressed CBP marker genes [54] (Leonhardt et al., 2004) were used to test the purity of cell preparations (see Results). ACTIN2 was used for an internal loading control. To amplify each CDPK-specific band, RT-PCR was performed with primer sets as indicated by arrowheads in (B) for 36 cycles. Plants were sprayed with water (−ABA) or 100 μM ABA (+ABA) 4 h before isolation of protoplasts and RNA extraction.
(B) Cartoon showing the T-DNA insertion positions in cpk3 and cpk6 T-DNA insertion alleles. PCR was performed with a left boarder primer of the T-DNA and a gene-specific primer, and the PCR products were sequenced to determine the T-DNA insertion positions. Arrowheads indicate primer locations for RT-PCR in (A) and (C). ATG and TGA indicate start and stop codons. White boxes indicate exons.
(C) RT-PCR confirmed that cpk3–1 and cpk6–1 alleles were disruption mutants. PCRs (32 cycles) were performed with primer sets as indicated in (B) (black arrowheads) in the left three panels. Transcripts of wild-type (WT) and cpk6–2 were examined with two sets of primers [white and black arrowheads in (B)] showing that cpk6–2 lacks exon 1 and that the cpk6–2 has 8% or less the mRNA level of wild-type based on densitometry analyses (n = 2). RNA was extracted from leaves of WT, homozygous cpk3–1, cpk6–1, and cpk6–2 single mutants, and the cpk3-1cpk6–1 double mutant.