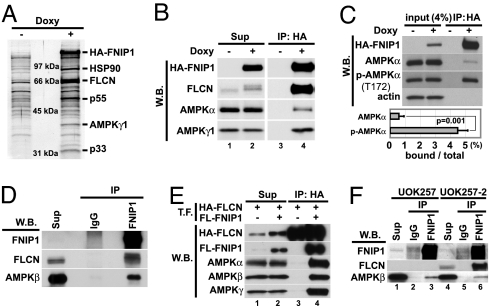
Fig. 4.
FNIP1 interacts with AMPK in vitro and in vivo. (A) FNIP1-interacting proteins were immunoprecipitated (IP) with anti-HA antibody from doxycycline-inducible HA-FNIP1-expressing HEK293 cells, separated by SDS/PAGE, transferred to PVDF membrane, stained with colloidal gold, and analyzed by mass spectrometry. The 40-kDa protein was identified as the γ-1 subunit of AMPK. Other interacting proteins were FLCN (67 kDa) and HSP 90 (90 kDa). (B) Cell lysates from HA-FNIP1-inducible HEK 293 cells cultured with and without doxy were immunoprecipitated with anti-HA antibody and blotted with antibodies, as indicated, showing that HA-FNIP1 and AMPK subunits interact. (C) Immunoprecipitates from HA-FNIP1-inducible HEK293 cells were blotted with antibodies as indicated. Band intensities were measured with the ImageJ program. The estimated amounts of AMPKα and phospho-AMPKα(T172) binding to FNIP1 were calculated by IP/[input (4%) × 25]. Results were from three independent experiments. Brackets, standard deviation. (D) HEK293 lysates were immunoprecipitated with anti-FNIP1 or control IgG. Immunoprecipitates were blotted with anti-FNIP1, anti-FLCN mouse monoclonal antibody, and anti-AMPKβ mouse monoclonal antibody. Endogenous FNIP1 and AMPK interact. (E) HEK293 cell lysates transfected (T.F.) with HA-FLCN alone or HA-FLCN and FLAG-FNIP1,were immunoprecipitated with anti-HA antibody and blotted with antibodies as indicated. All AMPK subunits were immunoprecipitated with FLCN in a FNIP1-dependent manner. (F) UOK257 cells (FLCN null) and FLCN-restored UOK257-2 cells were immunoprecipitated with anti-FNIP1 or control IgG and blotted with anti-FNIP1, anti-FLCNmAb, and anti-AMPKβ mAb. W.B., Western blotting; Sup, supernatant.