Abstract
Purpose
The matrix GLA (MGP) gene has been found to be among the 10 most highly expressed genes in the human trabecular meshwork (TM), and its expression is affected by conditions associated with glaucoma. Because MGP protein has been shown to play a key role in inhibiting calcification in cartilage and arterial vessels, MGP’s function in human TM was investigated.
Methods
Perfused TM tissue and primary human TM (HTM) cells originated from donors of nonglaucomatous eyes. MGP mRNA was assayed by relative quantitative and real-time PCR. AdhMGP recombinant adenovirus was generated by bacterial transposition. Western blot analyses were cross-reacted with MGP N-terminal- and conformational-specific antibodies. MGP/ BMP2 colocalization was analyzed by confocal microscopy. γ-Carboxylation activity was measured by incorporation of 14CO2into FLEEL synthetic peptide. Alkaline phosphatase (ALP) activity was used as a marker of osteogenic differentiation and a calcification precursor. Calcification was assessed by measuring direct calcium (o-cresolphthalein). Normalization was conducted with a telomerase probe (genomic DNA).
Results
HTM cells contained high levels of γ-carboxylase activity and were able to convert MGP to its active conformation. Overexpression of MGP in HTM cells reduced ALP activity in a model of BMP2-induced osteogenesis. MGP colocalized intra-cellularly with BMP2. HTM cells aged in culture exhibited increased calcium content, increased ALP, decreased normalized MGP expression and lower γ-carboxylase activity.
Conclusions
MGP protein is active and functions as an inhibitor of BMP2-induced ALP activity in the HTM cells. The human TM may undergo a calcification process with age. Inhibition of the calcification mechanism mediated by MGP could be used to regulate resistance and elevated IOP.
The outflow pathway tissue, composed of the TM and Schlemm’s canal, is responsible for the maintenance of physiological pressure inside the eye. This tissue performs its function by regulating the resistance to aqueous humor outflow. Failing to regulate aqueous humor resistance properly can result in an elevation of intraocular pressure (IOP), the major risk factor for the development of glaucoma.1,2 Glaucoma, an optic neuropathy characterized by the death of the retinal ganglion cells, is the second most frequent cause of irreversible blindness worldwide and the most common cause among African-Americans.3 The prevalence of glaucoma, particularly primary open-angle glaucoma (POAG), increases with age, affecting approximately 1% of people at age 40 and reaching approximately 4% of people by the age of 80.4
Over the years, several different mechanisms of the outflow pathway have been implicated in causing increased resistance to aqueous humor flow. Mechanisms such as secretion, phagocytosis, cell shape, cell adhesion, transport, and cell signaling have all been shown to have a role in outflow pathway function. It is also well established that an increase in extracellular matrix (ECM) deposition correlates with increased resistance and glaucoma.5
During the course of our past cDNA library and differential expression microarray studies, we have found that genes essential in cartilage and bone formation are abundant in the human TM and that some of them are modulated by elevated pressure.6 – 8 In particular, we have observed that the gene encoding matrix GLA protein (MGP) is among the 10 most highly expressed genes in our TM library7 as well as in subsequent expression profiles in which human TM from different sources was used.9,10 MGP was further found to be upregulated by an elevation of IOP lasting 2 to 4 days,8 a time when a homeostatic mechanism appears to be present in the human TM.11 In addition, MGP expression in HTM cells was downregulated by transforming growth factor-β1 (TGFβ1),8 a growth factor found elevated in patients with glaucoma.
MGP is a 14-kDa secreted protein found most abundantly in bone and cartilage. It was initially discovered in demineralized bovine bone matrix,12 but later was also found to have a key role in cardiovascular calcification.13 After MGP is synthesized, it undergoes a posttranslational modification in the endoplasmic reticulum (ER) where specific glutamic acid (Glu) residues are converted to γ-carboxylglutamic acid (Gla) by a vitamin K-dependent γ-carboxylase enzyme.12 This posttranslational modification induces a Ca2+-dependent conformational change in the Gla-containing region of the protein. It has been shown recently that this conformer of MGP is a binding protein for bone morphogenetic protein-2 (BMP2),14,15 a member of the TGFβ family of growth factors and a potent inducer of bone formation.16 It is believed that MGP acts as a negative regulator of BMP2 and inhibits its action as an inducer of calcification in the ECM.
MGP-knockout mice display extensive calcification of the arteries and cartilaginous tissues, and they die at approximately 6 weeks of age.17 This pathology can also be induced by depriving extrahepatic tissues of vitamin K.18 Together, these findings have established that MGP is a calcification inhibitor and suggests that the vitamin K modification of MGP is needed for this function of the protein.
Calcification of the arterial wall is a common occurrence in the pathophysiology of end-stage kidney disease, type 2 diabetes, and aging.19 In humans, a rare inherited disease, Keutel syndrome, characterized by excessive calcification of cartilage and stenosis of pulmonary arteries has been linked to a defective MGP gene.20 –22
The high abundance of MGP in the human TM, the association of aging with the development of increased outflow resistance and glaucoma, and the involvement of MGP in the calcification of soft tissues led us to hypothesize that MGP may play a similar role in the human TM. We considered that a similar process of calcification could contribute to the physiology of the human TM and to the regulation of IOP. To date, no such a process has been described in this tissue.
In this study, we investigated the function of MGP in the HTM cells. We wanted to know whether MGP existed in its fully γ-carboxylated active form in these cells and whether it worked as a calcification inhibitor by binding BMP2 to prevent unopposed activity of the growth factor. Because MGP has been known to have a role in the aged calcification of vascular smooth muscle cells (VSMCs), we further sought to determine whether aging HTM cells show the same signs of calcification as that observed in cells of the vascular system and whether there is differential gene expression of MGP with age.
We present evidence that MGP appeared to conserve its inhibition of calcification function in the HTM cells. We used replication-deficient adenoviruses encoding human BMP2 cDNA and found that we were able to induce calcification in HTM cells. Because alkaline phosphatase (ALP) is a well-established marker of early osteogenic differentiation, we evaluated ALP activity. ALP has been shown to be significantly elevated in committed osteoblasts23 and recently, calcification of human heart valve cells was seen to be dependent on ALP activity.24 In the current study, we found that ALP activity and calcification was elevated in HTM cells on BMP2 overexpression and that the increased ALP was counteracted by overexpression of MGP. In addition, we found that older HTM cells in culture undergo calcification. Our findings suggest for the first time the presence of the inhibition of an osteogenic calcification process in the HTM cells that is mediated by MGP and could affect the resistance to aqueous humor flow in the TM tissue.
Materials and Methods
Cells and Organ Cultures from Human Donor Eyes
Nonglaucomatous eyes from human donors were obtained within 40 hours of death from national eye banks (Lions Eye Bank of Oregon, Portland, OR, and National Disease Research Interchange, Philadel-phia, PA) after signed consent of the patients’ families. All procedures were in accordance with the tenets of the Declaration of Helsinki. For isolation of HTM cells, the TM from both eyes of a single individual were isolated from surrounding tissue by making incisions both anterior and posterior to the meshwork and removing it with forceps. The tissue was then cut into small pieces and treated with 1 mg/mL collagenase type IV (Worthington, Lakewood, NJ), as described.8 These primary nontransformed cells subsist for 9 to 10 passages. Experiments in this study were performed with cell lines HTM-39, -41, -42, -43, -50, and -63 (ages, 18 –35 years old). One cell line, HTM-63, was generated from the residual cornea rim after a surgical corneal transplant at the University of North Carolina Eye Clinic. Cells from this specimen were not treated with enzymes and were allowed to grow from the explant for a period of 4 weeks. On confluence, cells were treated as follows. Cells were maintained in improved minimal essential medium (IMEM; Biofluids, Rockville, MD) and supplemented with 10% fetal bovine serum (FBS), 50 μg/mL gentamicin (Invitrogen-Gibco, Carlsbad, CA) in a humidified 6% CO2 atmosphere. All cells were used at passages 4 to 6. These outflow pathway cultures comprise all cell types involved in maintaining resistance to flow, including cells from the three distinct regions of the TM plus cells lining Schlemm’s canal. Because most of the cells in these cultures come from the TM, they are commonly referred to as TM cells. Human VSMCs were obtained from the American Type Culture Collection (CRL-1999; ATCC, Manassas, VA). Human embryonic kidney (HEK) 293 cells were obtained from QBiogene (Montreal, Quebec, Canada).
For the organ cultures, whole donor eye globes were dissected at the equator; cleaned from the vitreous, iris, and lens; and mounted in the perfusion chambers, as described previously.25,26 Perfusion was conducted at a constant flow, using serum-free, high-glucose Dulbec-co’s modified Eagle’s medium (DMEM) or a specialized lens medium consisting of modified TC-199 prepared as described by Zigler and Hess.27
RNA Extraction, Reverse Transcription, and Quantification of MGP cDNA
RNA extraction was performed by resuspending cellular pellets or homogenized TM tissue in guanidine thiocyanate buffer and loading the solution onto a column (QIAshredder; Qiagen, Valencia, CA). The extraction was continued with a kit with on-column RNase-free DNase digestion according to the manufacturer’s recommendations (RNeasy Mini kit; Qiagen).
Reverse transcription (RT) reactions and follow-up normalized cDNA quantification were performed by either the relative quantitative reverse transcriptase polymerase chain reaction method (RQ-RT-PCR; Ambion, Austin, TX) or real time PCR (TaqMan; Applied Biosystems, Inc. [ABI] Foster City, CA).
The RQ-RT-PCR procedure was conducted as previously detailed,8 but with 1 μg total RNA as the starting material. Determination of the exponential PCR amplification cycle was conducted as described8 but using a 2.5-μL-aliquot RT reaction, 1 μL of the polymerase (5 U; SuperTaq; ABI), and MGP primers (forward: 5′-CGC CTT AGC GGT AGT AAC TTT GT-3′; reverse: 5′-TTT TCT TCC CTC AGT CTC ATT TG-3′). Amplification conditions were 94°C, 4 minutes and cycles of 94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 1 minute, with a final extension at 72°C for 4 minutes. PCR products were electrophoresed on a Tris-borate 1.5% agarose gel (SuperAcryl; DNA Technologies, Halifax, Nova Scotia, Canada). Quantification of the RQ-RT PCR products was accomplished by the use of an 18S rRNA classic primer-competimer set (Quantum RNA; Ambion), which reduces the abundant 18S amplification by the addition of competimers.8 Multiplex MGP and 18S PCR amplifications were performed at the predetermined linear cycle for the comparison conditions. MGP primers were designed to amplify a 296-bp fragment whose position in the gel was clearly separated from that of the internal 18S marker (489 bp). For relative quantification, reactions were repeated in triplicate at conditions in which the multiplex PCR products were all in the linear range. Band intensities were captured (ChemiDoc System, including Chemi-cooled charged-coupled device camera, PCI digitizing image acquisition board, EpiChemi II Darkroom with transilluminator; and Lab-Works Software; all from UVP, Upland, CA) and transferred to a spreadsheet for calculation of averages and standard errors.
For the real-time procedure, RT was conducted with approximately 0.5 μg of total tissue RNA in a 25-μL total volume of proprietary RT buffer containing random primers, dNTPs, and 62.5 U of RT enzyme with RNase inhibitor (Multiscribe MuLV in a high-capacity cDNA kit; ABI) according to the manufacturer’s recommendations (25°C 10 minutes, 37°C 2 hours). Fluorescently labeled probes and primer sets of selected genes (TaqMan; ABI) were purchased (TaqMan Gene Expression collection; http://www.allgenes.com; ABI). The MGP probe corresponded to sequences from exons 1 and 2 (Hs00179899_m1) and carbonic anhydrase II (CA2) probe corresponded to sequences from exons 2 and 3 (Hs00163869_m1). Reactions were performed in 20-μL aliquots using universal PCR buffer (NoAmpErase UNG; ABI), run on a sequence detection system (Prism 7700; ABI) and analyzed by SDS software. Changes (x-fold) were calculated by the formula 2−ΔCT where is CT the cycle at threshold and ΔCT is the CT of the assayed gene (MGP) minus the CT of CA2. TM tissue was dissected from the 50-hour perfused anterior segments of eyes of an 82-year-old white donor with no history of glaucoma.
Viral Vectors and Infection of HTM Cells
The AdhMGP vector carrying the full human MGP coding region was constructed in our laboratory by site-specific bacterial transposition, with an adenoviral system (Transpose-Ad QBIOgene). An MGP cDNA fragment (nucleotides 13-349, GenBank accession number M58549; http://www.ncbi.nlm.nih.gov/Genbank; provided in the public domain by the National Center for Biotechnology Information, Bethesda, MD) containing the coding sequence nucleotides 27-338, was obtained by PCR amplification of plasmid 296 from our human HTM1 library,7 using high-proof Taq polymerase and primers 5′-TGCAGGACGAAAC-CATGAAG-3′ (forward) and 5′-TTCCCTCAGTCTCATTTGGC-3′ (reverse). These primers were designed to contain KpnI and XbaI sites at their 5′ ends, respectively. The amplified insert was digested with KpnI/XbaI, purified, and cloned into a KpnI/XbaI-predigested pCR259 transfer vector (QBiogene) under the transcriptional control of the cytomegalovirus promoter-enhancer (CMV;pWX1). On sequencing confirmation, pWX1 was transfected into Transpose-Ad294-competent Escherichia coli cells which transposed the pWX1 expression cassette into the Adgenome plasmid, disrupting its LacZ gene. The resultant plasmid (pWX2) was purified, linearized with PacI, and transfected into early-passage HEK 293 cells to produce the recombinant AdhMGP. High-titer viral stocks were obtained by propagation in HEK 293 cells and purification by double binding CsCl density centrifugation, as described.28 The virus particle number was determined by measurement of their optical density at 260 nm using the formula 1 μg DNA = 2.2 × 1010 particles. The absence of contaminant wild-type viruses was tested in each stock by PCR amplification with E1A-specific primers 5′-TCG AAG AGG TAC TGG CTG AT-3′ and 5′-TGA CAA GAC CTG CAA CCG TG-3′.
The Ad5BMP2 vector was constructed by one of the authors (EOD) and has been described.29 It contains the full-coding cDNA of the BMP2 gene, also driven by the CMV promoter. The Ad5.CMV-LacZ (Ad. βgal) and Ad5.CMV-Null (AdNull) adenoviruses were purchased from QBiogene, grown, and purified in our laboratory.
For viral infection, HTM cells at passages 4 to 6 were grown to 70% to 80% confluence, washed twice with phosphate-buffered saline (PBS), and exposed to AdhMGP or/and Ad5BMP2 for 1 hour in 1 mL serum-free medium. After exposure to the virus, serum was added to 2% FBS and incubated overnight, and full medium containing 10% FBS was added the next day. Incubation continued at 37°C, and fresh medium was replaced every 48 hours.
Protein Extraction and Western Blot Analysis
HTM cell cultures were grown to confluence in 35-mm dishes. After infection, the cell monolayer from each well was washed twice with PBS and harvested in 200 μL lysis buffer (150 mM NaCl, 50 mM Tris [pH 7.4], 1 mM EDTA, 1% NP-40, 0.25% deoxycholate, 1× protein inhibitor cocktail; Roche, Indianapolis, IN; 1 mM phenylmethylsulfonyl fluoride [PMSF], and 1 mM NaF) with a cell scraper and incubated on ice for 15 minutes. Cells were disrupted by sonication (Microson Ultrasonic XL2000; Misonix, Farmingdale, NY) equipped with a 2.4 mm microprobe (Misonix) at setting three for five pulses. The sonicate was then centrifuged at 14,000g for 15 minutes at 4°C and pellets used as insoluble fractions. Pellets were solubilized with 100 μL 10× running buffer (1×: 25 mM Tris [pH 8.3] 192 mM glycine, and 0.1% SDS; Bio-Rad Laboratories, Hercules, CA) and stored at −80°C until use. For organ culture, the dissected, perfused TM was washed with PBS, resuspended in 100 μL lysis buffer, and homogenized in a sterile glass microtissue grinder (Kimble-Kontes, Vineland, NJ). Insoluble fractions were then recovered and treated as described for the HTM cells. TM tissues used in these experiments were obtained from anterior segments of the eyes (two male white donors 76 and 77 years of age) that had been perfused for 3 and 4 days, respectively.
Before electrophoresis, proteins extracts were mixed 1:2 (vol/vol) with loading Laemmli buffer (Bio-Rad) containing 5% β-mercaptoethanol and boiled for 5 minutes. Proteins extracts were separated by 15% SDS-PAGE gels and electrotransferred to a PVDF membrane using a mini transblot system (Bio-Rad) and manufacturer’s buffers. After blocking with 5% nonfat dry milk in 0.01 M Tris-HCl (pH 8.0), 0.2% Tween 20 for 2 hours, membranes were incubated overnight at 4°C with a rabbit polyclonal MGP-N30 (1:1000) or MGP-GLA30 (1:500). After treatment with anti-rabbit IgG secondary antibodies conjugated to horseradish peroxidase (1:5000; Pierce Biotechnology, Inc, Rock-ford, IL), immunoreactive bands were visualized by chemiluminescence (ECL Plus; GE Healthcare, Piscataway, NJ) and membranes exposed to light film (BioMax MR; Kodak, Rochester, NY).
Determination of γ-Carboxylase Activity
HTM cells were washed with PBS, scraped from the flask, pelleted, and resuspended in 250 mM sodium phosphate containing 0.5 mM KCl, 20% glycerol, 0.75% CHAPS buffer (pH 7.85; 3-[3-cholamidopropy-l]dimethylammonio-2-hydroxy-1-propanesulfonate). γ-Carboxylase activity was assayed as described31 as 14CO2incorporation into the synthetic peptide FLEEL. The reaction was triggered by adding chemically reduced vitamin K1H2 (100 μg/mL) to the assay mixture containing 5 mM dithiothreitol and 40 mM of FLEEL.
Immunocytochemistry
For immunocytochemistry, HTM cells were cultured on glass cover-slips precoated with poly-D-lysine, fixed, and fluorescently double-labeled for the BMP2 and MGP proteins. Cells were washed twice with warmed PBS, fixed with warmed 4% paraformaldehyde for 10 minutes at RT, washed twice with PBS, permeabilized with 0.1% or 0.3% Triton-X/PBS for 10 minutes, and blocked for 20 minutes with 10% donkey serum–PBS. For the BMP2-MGP detection, cells were again washed with PBS and incubated overnight at 4°C with goat anti-rat BMP230 and rabbit anti-bovine MGP,30 followed by an additional 1-hour incubation with a mix of Cy2-conjugated donkey anti-goat and Cy3-conjugated donkey anti-rabbit secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA). For the BMP2-βgal detection, cells were incubated overnight at 4°C with goat anti-rat BMP230 and monoclonal anti-β-galactosidase (Promega, Madison, WI) followed by 1 hour incubation with a mix of Cy2-conjugated donkey anti-goat (Jackson ImmunoResearch Laboratories) and 7-amino-4-methyl-coumarin-3- acetic acid (AMCA)-labeled horse anti-mouse secondary antibodies (Vector Laboratories, Burlingame, CA). All antibody solutions were made in 10% donkey serum–PBS and washes with PBS were performed between all incubation steps (3×, 5 minutes each). Cover-slips were mounted (20 μL Fluoromount G; Southern Biotechnology Associates, Birmingham, AL) and sealed with clear enamel. High-resolution fluorescence imaging was performed with a confocal laser scanning microscope (SP2 AOBS; Leica, Deerfield, IL), operated with argon (488 nm) and diode-pumped solid state (561 nm) lasers. Spectral detection was set to 505 to 530 nm for FITC filter and 580 to 680 nm for rhodamine at the Michael Hooker Microscope Facility (University of North Carolina). Confocal images were collected by using 20 × 0.7 numerical aperture [NA], 40 × 1.25 NA, and 63 × 1.4 NA objectives, and pinhole size was set to 1 Airy disc unit, yielding optical slices of approximately 1-, 0.45-, and 0.35-μm thickness respectively. Digital images were arranged with image-analysis software (Photoshop CS; Adobe, Mountain View, CA). Negative controls were run in parallel but were incubated in 10% donkey serum-PBS in place of the primary antibodies.
ALP Assay
ALP activity in HTM cells was measured with a fluorescence substrate system (AttoPhos AP; Promega). This system uses 2′-[2-benzothiazoyl]-6′-hydroxybenzothiazole (BBTP) as a substrate, which is cleaved by the ALP enzyme to produce inorganic Pi and BBT, a derivative that shifts the fluorescence spectra and results in lower background and higher sensitivity. In contrast to other commercial fluorescence or luminescence ALP assay systems, this system does not contain an endogenous ALP inhibitor. To determine ALP activity, HTM cells in 35-mm dishes were washed twice with PBS, scraped with 1 mL PBS, centrifuged, resuspended in 200 μL PBS and lysed by freeze-thawing three times. Different aliquots from the same cell extracts were used to measure ALP and genomic DNA. For the ALP, an aliquot was adjusted to 100 μL with PBS and incubated with 100 μL substrate (AttoPhos; Promega) at room temperature in a black 96-well plate in the dark. A parallel set of incubations was conducted with different dilutions (from 100 ng to 5 ng) of a purified ALP enzyme (Roche) to generate a standard curve. Control experiments containing PBS aliquots without enzyme were run in parallel. Fluorometric values were read usually after 20 minutes of incubation time in a fluorescence plate reader (Fluostar; BMG Labtechnologies, Durham, NC) with a 430-nm excitation and 555-nm emission filters. Fluorescence from the no-enzyme control sample was subtracted from each of the samples and ALP concentrations calculated by extrapolation on the standard curve and correction for aliquot volume.
Genomic DNA Extraction and Quantification
HTM cell genomic DNA was isolated (DNeasy Tissue Kit; Qiagen). Half of the harvested cell extracts (described earlier) were adjusted to 200μL with PBS and treated with 0.5 mg proteinase K for 10 minutes at 70°C in the presence of the manufacturer’s buffer (AL). The treatment was followed by the addition of 0.5 vol 100% ethanol and subsequent binding to a mini spin column (DNeasy; Qiagen). After repeated washes, DNA was eluted from the column with 400 μL of the manufacturer’s buffer (AE) and saved at −80°C until use. Yields were typically 4 to 5 μg per extraction.
Host-specific cellular DNA was quantified (Quantifiler Human DNA Quantification kit; ABI). Two microliters of each DNA sample was added to 10.5 μL of probe-primer and a 12.5-μL reaction mix in optical density tubes (TaqMan; ABI). The probe-primer in the mix hybridizes and amplifies the human RT telomerase in the host DNA plus an artificial internal template that is used as a fluorescence normalization control. Probes are fluorescent 5′FAM (telomerase probe) and fluorescent 5′VIC (control probe to artificial template). Samples were run in duplicate in a sequence-detection system (Prism 7700; ABI) and analyzed (SDS software; ABI). Cycling conditions were 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Each run included a serial dilution of human DNA standard (Quantifiler; ABI) as well as nontemplate, negative controls tubes. The number of genomes in each DNA sample was calculated from the standard curve generated by plotting the CT against each known concentration of the human DNA standards.
Alizarin Red Staining
The cells were grown in six-well plates, washed with PBS three times, and then fixed in cold 80% ethanol for at least 1 hour at 4°C. They were then washed in distilled water and exposed to fresh 2% alizarin red (pH 4.2; Sigma-Aldrich, St. Louis, MO) for 5 minutes (red-orange shows positive staining). They were subsequently washed with distilled water at least five times followed by one PBS wash for 15 minutes and photographed with a microscope-mounted digital camera (DP70; Olympus, Lake Success, NY).
Quantification of Calcium Deposition
HTM cells in six-well plates were washed three times with PBS and decalcified with 250 μL 1 N HCl at 37°C for 24 hours. Supernatants were concentrated in a centrifuge (Speed-Vac; Thermo Savant, Hol-brook, NY) and the calcium content determined colorimetrically (absorbance 570 nm) by the o-cresolphthalein complexone method (Calcium C-test; Wako Chemicals USA, Richmond, VA), according to the manufacturer’s recommendations. A standard curve was generated each time using the standards provided in the kit, and the Ca2+ concentration in the samples was calculated. Negative controls included equivalent samples with no cells. After decalcification, the cells were washed three times with PBS, scraped in 500 μL PBS and disrupted by sonication for 10 seconds at power level 3. DNA concentrations of cell extracts were determined (FluoReporter Blue Fluorometric dsDNA Quantitation Kit; Molecular Probes-Invitrogen). Aliquots were adjusted to 100 μL PBS and mixed with the kit’s Hoechst solution, according to the manufacturer’s recommendations. Each time, a parallel set of wells with different dilutions (from 1 μg–75 ng) of a standardized calf thymus DNA sample was included to generate a standard curve. Negative controls containing PBS aliquots without extract were run in parallel. Fluorometric values were read immediately after mixing in a fluorescence plate reader (Fluostar; BMG Labtechnologies) with 350-nm excitation and 450-nm emission filters. Fluorescence values from the negative control samples were subtracted from each of the samples and DNA concentrations calculated by extrapolation on the standard curve and correction for aliquot volume. The calcium content of the cell supernatant was normalized by DNA content of the cell extract.
Results
MGP mRNA and Protein in TM Cells and Tissue
We had previously shown that the number of MGP clones in a human TM library from a single donor was among those of the 10 most abundant genes.7 Our results were subsequently confirmed on human TM libraries from pooled adult individuals10 and pooled infant primary HTM cells.9 In addition, we and others have shown that the expression of the MGP gene is altered in the TM by insults known to be associated with glaucoma, such as elevated IOP insult of 2 to 4 days8 or 7 days (Borras T, unpublished results, 2004), TGBβ18 and dexamethasone (Borras T, unpublished results, 2004). To get a further insight on the relative abundance of MGP mRNA in the human TM, we used the more sensitive method of real-time PCR (TaqMan; ABI) and compared MGP expression with that of a gene shown to be present only once in one of the three existing libraries (CA2). RNA was extracted from TM-perfused tissue from a new donor and MGP and CA2 expression measured as indicated in the Methods section. MGP expression was 191 ± 19.2 times that of CA2 (n = 4, P = 0.00002; Fig. 1, top).
Figure 1.
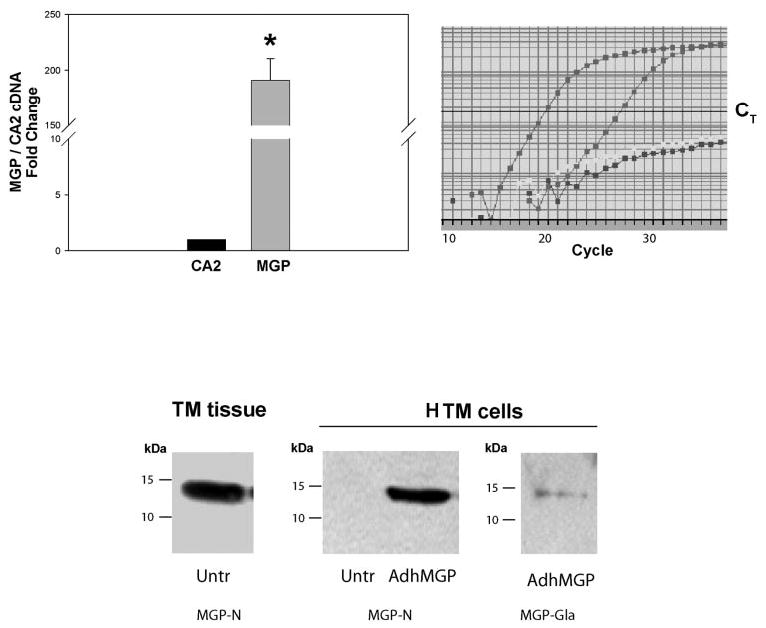
Characterization of MGP mRNA and protein in human intact TM tissue and HTM cells. Top: Relative MGP mRNA abundance. RNA from the pooled TMs from perfused anterior segments of an 82-year-old white donor, was reversed transcribed and identical aliquots amplified with specific probe-primers for MGP (Hs00179899_m1) and CA2 (Hs00163869_m1) (real-time PCR). The expression of MGP was compared with that of CA2 and expressed as x-fold change ± SEM. Right: a representative graph from one reaction (left curve: MGP; middle curve: CA2; right curve: nontemplate control for MGP and CA2). *P ≤ 0.00002, n = 4. MGP cDNA abundance was significantly higher than that of CA2. Bottom: MGP protein detection by Western blot analysis. Insoluble proteins extracted from a single TM from perfused anterior segment (77-year-old white donor; left) and HTM cells (right) either untreated or infected with recombinant adenovirus AdhMGP (650 VP per cell). Proteins were separated on 15% SDS-polyacrylamide gels, transferred to PVDF membranes and incubated with rabbit polyclonal MGP antibodies.30 MGP-N is an N-terminal peptide antibody that recognizes total MGP. MGP GLA is a GLA-peptide antibody that specifically recognizes fully γ-carboxylated MGP. MGP was detected in perfused intact TM tissue but not in untreated HTM cells. Both, total MGP and carboxylated MGP were found in AdhMGP-infected HTM cells.
To determine the presence of the MGP protein, we performed Western blot analyses with protein extracts from perfused organ cultures from single individuals as well as primary HTM cells. Using the antibody raised against the 32-residue N-terminal peptide of the protein,30 MGP was detected in the insoluble protein fraction from the perfused intact tissue, and it was not detected on primary HTM cultured cells (Fig. 1, bottom). However, infection of the primary cells with Adh-MGP, the recombinant adenovirus carrying MGP cDNA, yielded an MGP recombinant protein that appeared indistinguishable from the endogenous form. In addition, extracts from the infected cells cross-reacted with the conformational specific MGP-GLA antibody (Fig. 1, bottom). Furthermore, the carboxylase activity in untreated cultured primary cells corrected by the non VitK control, was 2440 ± 57 cpm of the substrate per microgram total protein. This high level of car-boxylase activity is an indication of the ability of the HTM cells to convert inactive MGP to its active, BMP2-binding form.
ALP Activity and Calcification of HTM Cells Was Induced by Overexpression of BMP2
MGP function has been defined as that of an inhibitor of calcification. To study the potential effect of MGP in HTM calcification in vitro and to determine whether established calcification inducers had similar properties in the cells of the outflow tissue, we choose BMP2, a factor known to induce calcification in vascular cells.32 HTM-63 cells at passage 4 seeded at 70% confluence were infected once with 2.1 × 108 viral particles (VP; ~250 VP/cell) of Ad5BMP2, a replication-deficient adenovirus carrying the human BMP2 cDNA. Parallel wells remained untreated, and all cells were incubated for 1, 3, and 5 days, respectively. At the end of the incubation period, the media were removed and the cells washed, harvested, and resuspended in PBS. Samples were split in two, and ALP measurements were assayed and normalized as indicated in Materials and Methods. Special care was taken that the DNA assay used for normalization (Telomerase TaqMan probe; ABI) detected exclusively the host, but not the introduced, viral DNA. As an indication of a correlation between ALP activity and calcification, direct cellular calcium measurements were conducted in one parallel experiment. HTM-63 cells were infected with Ad5BMP2 under the same conditions and the calcium levels evaluated and normalized. At 3 days after infection, the calcium level of the Ad5BMP2-infected cells was 1007.8 ng Ca/μg DNA, whereas that of the untreated cells was 143.9 ng Ca/μg DNA.
Results shown in Figure 2 indicate that BMP2 induced significant, time-dependent ALP activity in the HTM cells. The normalized endogenous ALP was 16.6 ± 1.6 ng/μg genomic DNA (n = 4; P ≤ 0.017) at 1 day after infection, increased to 37.5 ± 4.5 ng/μg genomic DNA (n = 4; P ≤ 0.0005) at 3 days and reached 50.3 ± 4.3 ng/μg genomic DNA (n = 4; P ≤ 0.00006) at 5 days, which was the last point assayed. Experiments were repeated at least four more times with a different cell line (HTM-41) with comparable results. Although values had a wider range, increases of normalized ALP were also obtained on infection with 10 times the number of Ad5BMP2 VP. Typically, at the higher VP concentration, ALP activity increases were four to eight times those in the uninfected cells at 3 days after infection, and the increase reached 8.5- to 140-fold after 7 days.
Figure 2.
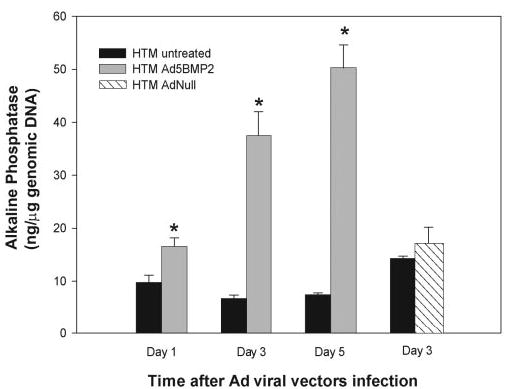
Induction of ALP activity in HTM cells by overexpression of human BMP2. HTM cells at passage 4 were infected once with a recombinant adenovirus carrying the human BMP2 gene (Ad5BMP2, 250 VP per cell). Control cells were infected with a replication-deficient adenovirus carrying no transgene (AdNull, 250 VP per cell). One, 3, and 5 days after infection, harvested cells were divided into aliquots and evaluated for endogenous ALP and nonviral, host genomic DNA content. Parallel, untreated, and viral control infected cells were analyzed in the same manner. ALP data were normalized to cell number, represented by total genomic DNA (mean ± SEM, n = 4). *P ≤ 0.017 (day 1), P ≤ 0.0005 (day 3), P ≤ 0.00006 (day 5). Normalized ALP levels in the AdNull-infected cells were not significantly different from those in untreated cells (3 days; P ≤ 0.41). BMP2 induced a significant, time-dependent increase of ALP activity in the HTM cells.
To control whether the increased ALP activity was the result of the core of the adenoviral vector, in a parallel experiment we infected HTM-63 cells with an AdNull viral vector, which carries no transgene. HTM cells were infected with the same number of VP as the Ad5BMP2 virus (250 VP/cell) and assayed for ALP at 3 days after infection. The normalized ALP activity of the AdNull-treated cells was not significantly different from that of the untreated control (17.1 ± 3.3 ng/μg genomic DNA [n = 4] versus 14.3 ± 0.4 ng/μg genomic DNA [n = 4] respectively, P ≤ 0.41; Fig. 2). In the same experiment, the ALP activity of Ad5BMP2-infected cells was 83.6 ± 15.4 ng/μg genomic DNA (n = 4; P ≤ 0.0002, compared with AdNull).
These findings indicate that the cells of the outflow tissue can undergo a process of calcification in vitro that appears to be similar to the calcification process described in vitro and in vivo for calcification of arteries and cartilage.17,32
MGP Decreased BMP2-Induced ALP Activity in HTM Cells
Several investigators have shown in other systems that MGP binds and modulates the BMP2 protein.14,15 To determine whether MGP function(s) in the HTM primary cells would also include the prevention of an increased ALP activity potentially indicative of a calcification process, we compared the ALP activity observed after infection with Ad5BMP2 with that obtained after co-infecting HTM cells with adenoviruses encoding BMP2 and MGP proteins.
HTM-41 cells at passage 6 and 80% confluence were infected with either 2.1 × 108 VP of Ad5BMP2 alone or in combination with 7.8 × 108 VP or 1.6 × 109 VP of AdhMGP (VP ratio ~1:4 and 1:8, respectively). Parallel wells were left untreated or were infected with the same number of particles of AdhMGP alone as controls. Cells were incubated for 3 days, assayed, and normalized for ALP and genomic DNA, as indicated earlier.
At 3 days after infection, samples infected with Ad5BMP2 exhibited a 366.4% ± 153% change in ALP ng/μg DNA over control samples (n = 7, P ≤ 0.002; Fig. 3). In contrast, samples co-infected with Ad5BMP2-AdhMGP (ratio 1:4) exhibited a 90.4% ± 72% change over the control (n = 7). This value was significantly lower than that of Ad5BMP2 (P ≤ 0.0003) and not significantly different from the uninfected control (P ≤ 0.30). Likewise, co-infections at a ratio of 1 to 8 resulted in a 66.4% ± 71% change over control (n =7), which was significantly lower than that of Ad5BMP2 (P ≤ 0.0005), and not significantly different from the uninfected control (P ≤ 0.70). Cells infected with AdhMGP alone at the same VP used in the co-infections (1:4 and 1:8) resulted in a change of 28.1% ± 25.6% over the control (n = 7; P ≤ 0.001 compared with Ad5BMP2, P ≤ 0.29 compared with uninfected control) and 15% ± 21% (n = 2; P ≤ 0.02 compared with Ad5BMP2, P ≤ 0.65 compared to uninfected control).
Figure 3.
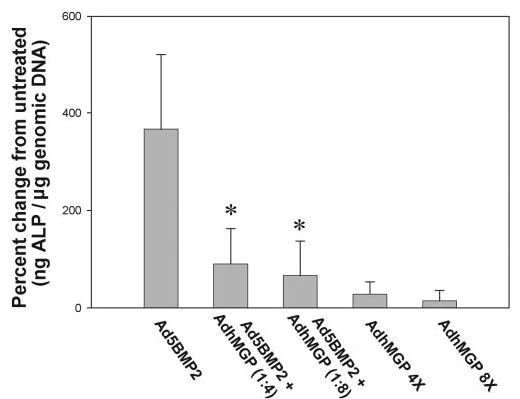
Reduction of BMP2-induced ALP activity in HTM cells by overexpression of MGP. Nontransformed HTM cells at passage 6 were infected with Ad5BMP2 alone (250 VP per cell) or combined with AdhMGP (VP ratios, ~1:4 and 1:8, respectively). Untreated cells and cells infected with the same number of AdhMGP VP alone were used as the control. Three days after infection, harvested cells were divided into aliquots and evaluated for endogenous ALP and nonviral, host genomic DNA content. ALP levels were thus normalized to cell number, represented by total genomic DNA. Results are expressed as the percentage change of normalized endogenous ALP in infected compared with uninfected samples (mean ± SEM). *P ≤ 0.0005, n = 7. MGP alone did not induce significant ALP activity and inhibited the increased ALP effect induced by BMP2 in the HTM cells.
As an indication of a correlation between ALP activity and calcification, direct cellular calcium measurements were conducted in one parallel experiment. HTM-63 cells were infected with Ad5BMP2 and AdhMGP (ratio, 1:4) under the same conditions and calcium levels, evaluated, and normalized. At 3 days after infection, the calcium level of the Ad5BMP2-infected cells was 1007.8 ng Ca/μg DNA, whereas that of the co-infected well was 230.7 ng Ca/μg DNA.
These results indicate that overexpression of MGP at a dose corresponding to infection of 1000 VP/cell does not by itself induce an increase in ALP activity in the HTM cells. Furthermore, at the same dose, MGP does function to inhibit the calcification process effect induced by BMP2 in the cells of the outflow pathway. These results also imply that the high carboxylase activity observed in these cells contributes to the conversion of the inactive MGP protein to its active form, which in turn allows the protein to engage in additional functions.
Intracellular Colocalization of Transferred BMP2 and MGP into HTM Cells
No positive staining was obtained in any of the uninfected HTM cells, including those with positive MGP RNA, indicating that the amount of endogenous proteins was insufficient to be detected by immunofluorescence. Thus, to investigate whether BMP2 and MGP would colocalize in the HTM cells, we co-infected these cells with recombinant adenoviruses encoding both proteins (~2000 and ~650 VP/cell, respectively). Forty-eight hours after infection, cells were fixed with 4% paraformaldehyde and incubated overnight with a mix of rabbit anti-bovine MGP (1:200) and goat anti-rat BMP2 primary antibodies (1:200). Detection was achieved with Cy3-conjugated anti-rabbit (1:200) combined with Cy2-conjugated donkey anti-goat (1:200) secondary antibodies. Confocal analyses of a representative field are shown in Figure 4, top. Micrographs obtained on individual filter channels showed a concentrated perinuclear, endoplasmic reticulum (ER) staining of MGP and a somewhat more overall cytoplasmic staining of BMP2. High-magnification merged images showed colocalization of the two proteins occurring around the nuclei, mostly coinciding with the location of MGP on the ER. Although most of the MGP in the cells seemed to colocalize with BMP2, only some of the BMP2 staining colocalized with MGP.
Figure 4.
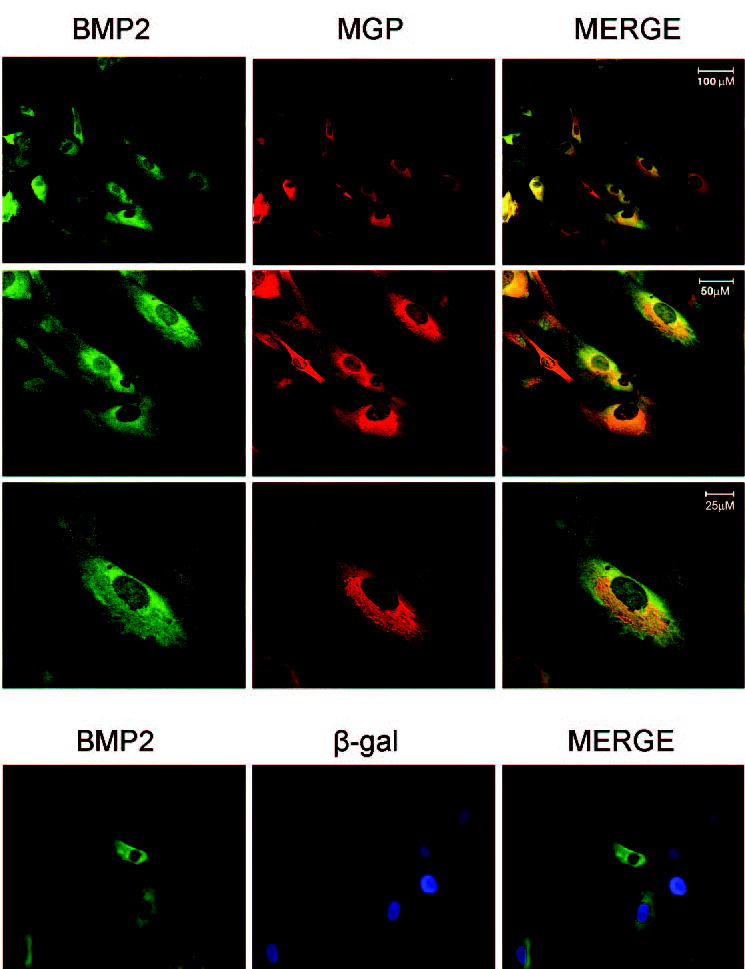
Colocalization of recombinant BMP2 and MGP in HTM cells. Top: HTM cells were co-infected with 7.8 × 108 VP of Ad5BMP2 and 2.1 × 109 VP of AdhMGP expressing the BMP2 and MGP proteins. At 48 hours after infection, the cells were fixed, permeabilized, and processed for immunofluorescence. Cells were double-stained with a rabbit anti-bovine MGP and goat anti-rat BMP2 primary antibodies followed by Cy3-conjugated donkey anti-rabbit and Cy2-conjugated donkey anti-rat secondary antibodies. Fluorescent images were obtained by confocal laser scanning microscopy with FITC (BMP2) and rhodamine (MGP) channels and objectives ×20 for the first row: optical slice thickness, 1.0 μm, ×40 for the second row: optical slice thickness, 0.45 μm; and ×63 for the third row: optical slice thickness 0.35 μm. Scale bars, 100, 50, and 25 μm, respectively. Bottom: HTM cells co-infected with equivalent VP of Ad5BMP2 and Ad. βgal expressing the BMP2 and β-galactosidase proteins. Cells were double-stained with goat anti-rat BMP2 and monoclonal anti-β-galactosidase primary antibodies followed by Cy2-conjugated donkey anti-rat and AMCA-labeled secondary antibodies. Fluorescent images were obtained with a fluorescence microscope under green (Cy2-BMP2) and blue (AMCA-βgal) channels. High-magnification merged images show colocalization of the BPM2 and MGP proteins in the perinuclear area of the cells, mostly coinciding with the ER. A control-viral transduced protein (βgal) did not colocalize.
Assessment of specificity for colocalization of two adenovirus-transduced proteins was performed by co-infecting Ad5BMP2 with a recombinant adenovirus carrying the reporter gene LacZ, which encodes the γ-galactosidase enzyme. Immunocytochemistry was conducted in a similar manner with the BMP2 primary antibody combined with a monoclonal anti-γ-galactosidase antibody (1:200) and followed by adding a horse anti-mouse AMCA-labeled antibody (1:50) to the BMP2 secondary. Analyses by standard fluorescence microscopy showed no colocalization.
These results suggest that the two proteins colocalize and could bind to each other before exiting the cell. The vitamin K cycle is embedded in the interior of the rough ER and the γ-carboxylase binding to vitamin K-dependent proteins occurs in the ER.33 A potential binding of a mature MGP would be in good correlation with the functional results obtained in this study and may indicate that MGP sequesters some of the BMP2 protein in the HTM cell and thus inhibits its potential for inducing a calcification process.
Aging HTM Cells Undergo In Vitro Calcification
Because glaucoma is an aging disease and because MGP inhibited ALP activity in primary HTM cells, we next looked at the calcification process in aged cultures of these cells. Primary HTM cells from passages 5 to 6 were grown to confluence and feed twice a week for 12 weeks. HTM cells maintained in culture for >30 days began to exhibit a distinct morphology, which was characterized by cell retraction, agglomeration and formation of multicellular nodules. Because these structures have been reported to be associated with calcification in VSMC,34 we determined whether they would also represent calcification nodules in the HTM cells. On staining with alizarin red, a well-established calcification marker, the observed nodules showed the red-orange color indicative of the positive staining, whereas younger cells from the same cell line were negative (Fig. 5, top). To further confirm and compare phenomenon seen on HTM cells with that published for VSMCs, we conducted a similar experiment with VSMCs kept in the incubator under identical conditions. Nodules formed by the VSMCs were indistinguishable from those observed on the HTM cells (Fig. 5, bottom).
Figure 5.
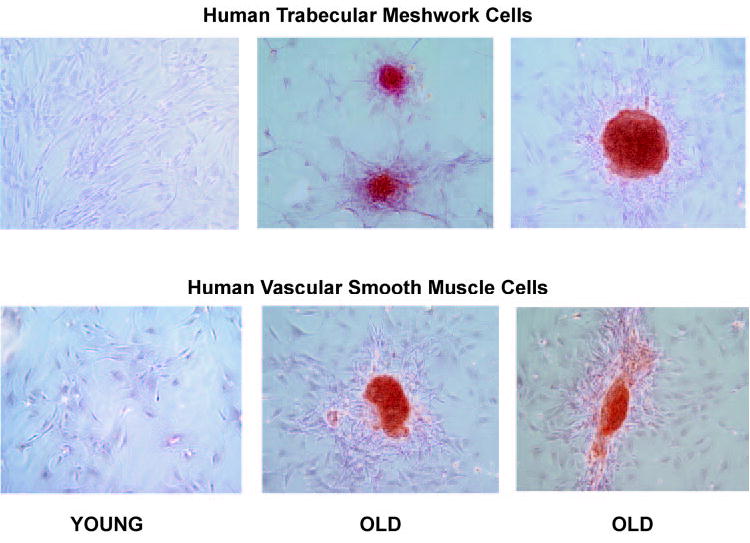
Formation of calcified multicellular nodules in old HTM cells. HTM passage 6 and VSMCs after 1 week (young) cells and 12 weeks (old) in culture stained with alizarin red. Old HTM cells formed multicellular nodules that showed positive red-orange staining for calcification. HTM calcification nodules were indistinguishable from those formed by VSMCs in the same conditions. Original magnification, ×100.
To further corroborate that the morphology observed is associated with an increase in calcification in the old HTM cells, we performed two additional assays, a chemically direct cellular calcium measurement, and a biological one, which assesses the activity of endogenous ALP. Both assays were normalized to the amount of total cellular DNA, either by Hoechst or real-time PCR. For proper ALP determination, care was taken to avoid the use of ALP reporter kits that happen to contain an inhibitor for the endogenous ALP enzyme.
For assaying levels of cellular calcium, HTM-41 young cells were at passage 5 and cultured for 7 days. HTM-41 old cells, originating from passage 7, were cultured for 30 days. Results showed that old cells had significantly higher calcium levels than young cells with values of 92.1 ± 2.4 ng Ca/μg DNA and 29.3 ± 2.7 ng Ca/μg DNA, respectively (n = 3 each set; P < 0.00006; Fig. 6, top). The experiment was repeated with HTM-63 cells, a cell line derived from a different individual, with results of 33.7 ± 2.5 ng Ca/μg DNA (old cells, passage 8 incubated for 30 days, n = 6) and 14.2 ± 1.8 ng Ca/μg DNA (young cell passage 5, 7 days, n = 5; P < 0.0002).
Figure 6.
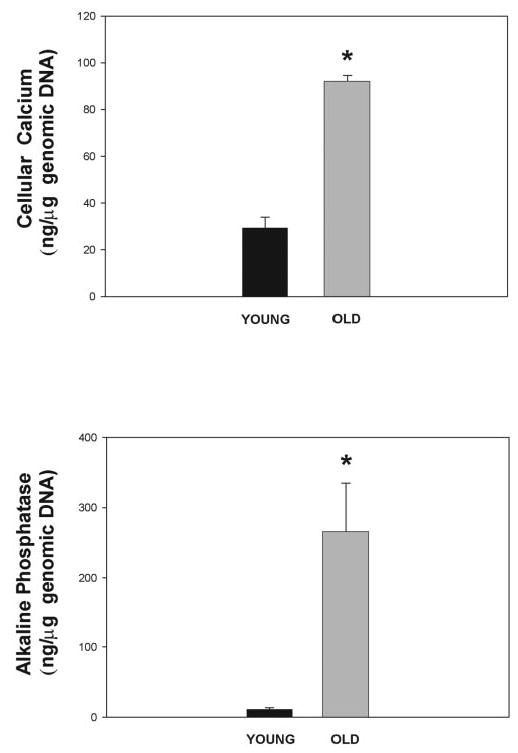
Calcification measurements in old HTM cells. Top: HTM cells passage 6 after 1 week (young) and 4 weeks in culture (old) were decalcified with 1 N HCl and their calcium content determined using the o-cresolphthalein method. DNA content was measured from cell extracts by Hoechst’s fluorescence. Results are shown as cell calcium levels normalized to the number of cells, represented by total genomic DNA (mean ± SEM). *P ≤ 0.00006 (n = 3 each). Bottom: HTM cells passage 4 to 6 after 1 week (young) and 4 to 12 weeks (old) in culture were harvested, divided into aliquots and evaluated for endogenous ALP and genomic DNA by real-time PCR. ALP levels were thus normalized to the number of cells, represented by total genomic DNA (mean ± SEM, n = 6 for young and n = 5 for old cells). *P ≤ 0.002. Old cells had significantly higher calcium and ALP levels than young cells.
Normalized levels of endogenous ALP were also significantly higher in old cells, correlating well with the direct calcium results. Figure 6 (bottom) represents values obtained with young (passages 4 – 6, 7 days in culture) and old HTM-41 (passage 7, 4 –12 weeks in culture). The ALP concentrations for the young cells were 10.8 ± 2.70 ng/μg DNA (n = 6; P ≤ 0.002), and they reached 266 ± 69 ng/μg DNA (n = 5) in the old ones. The ALP determinations were repeated in HTM-50 and HTM-42 originated from two additional individuals and normalized to total protein. Young cells showed ALP levels of 0.3 ng/μg protein (n = 3) versus 1.3 ng/μg protein in the old ones (n = 1).
Aging HTM Cells Exhibit Reduced Expression of MGP and Lower γ-Carboxylase Activity
Because a conformational active MGP was known to be key in the development of the calcification processes in the bone and vascular systems, and because MGP had been shown to be relevant in outflow tissues,7–10 we next investigated whether the gene encoding this protein was differentially expressed in aging HTM cells. HTM-41 cells at passage 5 were compared with those at passage 9 maintained in culture for 8 weeks. Total RNA was extracted, reverse transcribed, and amplified with MGP-specific primers and an 18S primer-competimer mix at the predetermined exponential range of 29 cycles for both genes and both samples. Samples were analyzed by gel electrophoresis. On normalization, values obtained from captured gel bands were 80,182 ± 2,849 integrated optical density (IOD) units for the young RNA and 54,371 ± 4,615 IOD for the old sample (n = 3 each set, P ≤ 0.009; Fig. 7). These results indicated that there is a significant 47.5%, downregulation of the MGP gene with age, and that this decreased expression appears to correlate with the increased calcification observed in the old cells.
Figure 7.
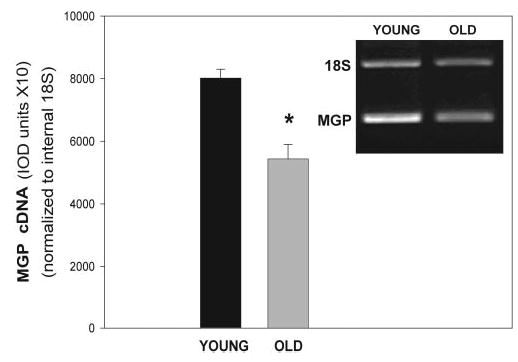
Normalized expression of the MGP gene in old HTM cells. MGP transcription of HTM cells of passage 5 after 1 week (young) and 8 weeks (old) in culture was measured by RQ-RT PCR. Multiplex amplifications were performed at the predetermined exponential 29 cycles and an 18s RNA primer-competimer ratio of 1:9. Expression of MGP was normalized against 18s RNA (mean ± SEM, n = 3 each). Inset: representative 1.5% Tris-borate agarose gel. *P ≤ 0.009.
In addition to the presence of MGP mRNA and its downregulation in old cells, inhibition of the calcification process led by this gene requires a posttranslational modification of the encoded protein, which converts the inactive into the active form of the protein. This modification occurs by the conversion of the amino acid Glu to GLA by the vitamin K-dependent γ-carboxylase enzyme. Therefore, we determined the presence of this enzyme in young and old HTM cells. HTM-41 cells of passages 5 (young) and 7 maintained for 12 weeks (old) were washed with PBS, scraped from the flask, pelleted, and prepared for the γ-carboxylase assay. Results of assays with young HTM samples showed an average activity of 2006 ± 50 cpm/ mg, whereas those from old cells amounted to 1164 ± 30 cpm/mg (n = 2 each set, P ≤ 0.005; Fig. 8). These results indicate that HTM cells have significant vitamin K-dependent carboxylase activity, and that this activity is reduced in these cells with aging. The carboxylase activity of the young HTM cells was found to be comparable to the activity measured in smooth muscle cells from rat aorta (Wallin R, unpublished observations, 2004), a fact that reinforces the relevance of the calcification process in the outflow tissue.
Figure 8.
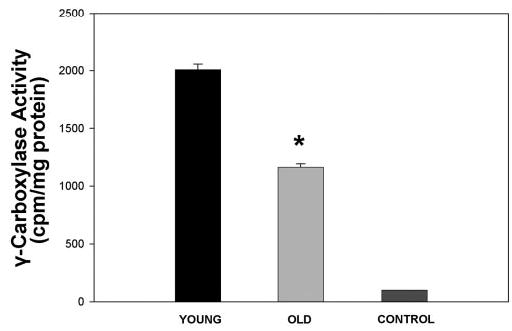
γ-Carboxylase activity of young and old HTM cells. γ-Carboxylase activity of HTM cells passage 5 after 1.5 weeks (young) and 12 weeks (old) in culture was measured. Activity is presented as 14CO2 incorporation into the synthetic γ-carboxylase peptide substrate FLEEL (14C-FLEEL) and is expressed as the mean ± SEM (n = 2 each). Control: 3-carboxylase activity triggered with chemically reduced Vit.KH2.Young HTM cells exhibited high γ-carboxylase activity, which was significantly reduced in old cells (*P ≤ 0.005).
Discussion
High-throughput sequence of cDNA libraries and microarray analyses comparing human TM gene expression under different conditions have opened the door to the discovery of potential new genes and functional mechanisms. One such finding has been the potential relevance and role of the MGP protein in the human TM, the tissue responsible for controlling the resistance of the aqueous humor flow in the eye. MGP has been recently considered of great relevance in maintaining the soft status of the cartilage tissue and to be necessary for preventing arterial calcification. Surprisingly, the gene encoding this protein has also been shown to be highly expressed in human TM. In addition, the expression of the MGP gene in the outflow tissue has been reported to be affected by conditions well established to be associated with the development of glaucoma, such as different time-period insults of elevated IOP, TGFβ1, and dexamethasone. In all cases, these insults altered expression of MGP in the HTM cells. In this study, we first confirmed that not only the mRNA, but the encoded MGP protein is present in the human TM tissue. As frequently occurs with other eye proteins (such as lens crystallins and TM myo-cilin) MGP expression was downregulated once the cells were started in cell culture conditions.
Although genes are known to have different functions in different tissues, to date, the most common function attributed to MGP is that of inhibition of calcification. Cells of the outflow tissue are not uniform and include endothelial-like cells which have been described as being of neural crest origin, as well as cells from Schlemm’s canal which are of vascular origin. Currently, there are no reports in the literature of trial assays of calcification in the human TM tissue. At the electron microscopy level, there are extensive reports of the presence of electron-dense materials, called sheath-derived plaques, whose presence is significantly increased in the TM of glaucomatous eyes.5 However, it is unknown whether these structures are associated with some form of calcification.
In this study, we found that MGP expression in the TM correlated with the calcification process. The carboxylase activity, the normalized ALP and the expression of MGP were all reduced on HTM cells allowed to age in the incubator for more than 4 weeks. In addition, during the aging procedure, there was a profound change in TM cell morphology. Cells aggregated and formed multicellular nodules that were very similar to those formed by VSMCs in similar conditions. Our results evaluating calcification by three essentially different assays (morphologic, chemical, and biological) indicate that cells of the HTM undergo a calcification process with age that is similar to that observed in cells of the vascular system.
These changes are consistent with proposed additional effects of MGP on morphogenesis through BMP2 regulation.32 Patients with Keutel syndrome (linked to MGP mutations) have not only abnormal diffuse cartilage calcification but other symptoms associated with multiple morphogenetic features, including facial deformations and developmental delay.22 Results from other investigators have also suggested that nodule formation precedes calcification and that this morphogenesis change involves a separate process.35 Of interest, a recently reported 6-year-old patient with Keutel had a sudden loss of vision in both eyes at age 3 and had bilateral optic nerve atrophy.22
It was somewhat unexpected that overexpression of BMP2 caused increased ALP activity and calcification in HTM cells. The BMP gene family has been studied in the TM. The BMP2, -4, -5, and -7 genes were expressed in cultured HTM cells obtained from different individuals.36 BMP2 was expressed in specimens from an early age, as well as in those from older individuals, and members of the BMP family have been implicated in normal eye development.37–39 In particular, a heterozygous BMP4 knockout mouse was reported to have anterior segment dysgenesis and elevated IOP.40 In addition to providing us with a workable osteogenic model, our findings of BMP2-induced ALP activity and calcification in the TM appear to support the notion that BMP2 may function in the eye tissue in a manner similar to its function in the vascular system, where it is critical for cardiovascular development in the embryonic stage but contributes to calcification during the adult stage of the individual.
The observed inhibitory effect of MGP on BMP2-induced ALP activity in the HTM cells is intriguing. It has been reported that MGP can be a conditional enhancer or inhibitor of BMP2-induced calcification in calcifying vascular cells.32 In the TM, the two BMP2-MGP relative concentrations studied (based on overexpression of the number of VP carrying both proteins) included four and eight times higher MGP levels. Both concentrations resulted in inhibition of BMP2-induced ALP activity. BMP2 belongs to the same superfamily of TGFβ growth factors, whose correlation with the TM on glaucomatous conditions is well referenced.41,42 Previous results from our laboratory8 and from microarray analysis (Paul Russell, National Institutes of Health, National Eye Institute, Bethesda MD, personal communication, 2004) showed that MGP mRNA is downregulated by TGFβ1 in the TM cells. Recently, however, MGP expression has been reported to be induced by treating bovine aortic endothelial cells (BAECs) with similar concentrations of TGFβ1.43 This different direction of the TGFβ1 effect observed in BAEC and HTM on MGP could be a reflection of the different cell types or even of the gene selected for normalization; but, in either case, it seems to be consistent with a functional interaction of the two molecules. Given the association of TGFβ with glaucoma,41,42 the question of whether MGP mediates this correlation by interfering with TGFβ1 remains to be resolved. In VSMCs it has been suggested that MGP may promote fibrosis though modulation of TGFβ1 activity.43,44
The elucidation of the role(s) of MGP in the TM and its contribution to regulating elevated pressure are not yet clear. In addition to the inhibition of calcification precursors observed here, MGP could also be involved in cellular morphogenesis, fibrosis, and maintenance of TM architecture at different stages of development. The high abundance of this protein as well as its response to glaucomatous insults are indicative of its physiological role in the TM. Altogether, our results in vitro support a role for MGP in modulating a calcification process that had not been previously investigated in the TM tissue. Continuation studies to verify this finding in an intact tissue and in vivo setting are needed. Electron microscopy and x-ray energy-dispersive microanalyses (in progress) would help to determine the presence of calcified deposits in the TM under normal and pathologic conditions. An underlying mineralization process would be key in regulating the resistance of the tissue to aqueous humor outflow and perhaps contribute to the development of glaucoma.
Acknowledgments
The authors thank Jason L. Vittitow for helpful discussions, Michael Chua for assistance with confocal microscopy, and Allison M. Eaton for technical support.
Footnotes
Disclosure: W. Xue, None; R. Wallin, None; E.A. Olmsted-Davis, None; T. Borrás, None
Supported by National Eye Institute Grants EY11906 (TB) and EY13126 (TB); and the Research to Prevent Blindness.
References
- 1.Anderson DR. Glaucoma: the damage caused by pressure. XLVI Edward Jackson memorial lecture. Am J Ophthalmol. 1989;108:485–495. doi: 10.1016/0002-9394(89)90423-6. [DOI] [PubMed] [Google Scholar]
- 2.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 3.Sommer A, Tielsch JM, Katz J, et al. Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans. The Baltimore Eye Survey. Arch Ophthalmol. 1991;109:1090–1095. doi: 10.1001/archopht.1991.01080080050026. [DOI] [PubMed] [Google Scholar]
- 4.Tielsch JM, Katz J, Singh K, et al. A population-based evaluation of glaucoma screening: the Baltimore Eye Survey. Am J Epidemiol. 1991;134:1102–1110. doi: 10.1093/oxfordjournals.aje.a116013. [DOI] [PubMed] [Google Scholar]
- 5.Lütjen-Drecoll E, Shimizu T, Rohrbach M, Rohen JW. Quantitative analysis of ‘plaque material’ in the inner- and outer wall of Schlemm’s canal in normal and glaucomatous eyes. Exp Eye Res. 1986;42:443–455. doi: 10.1016/0014-4835(86)90004-7. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez P, Zigler JS, Jr, Epstein DL, Borrás T. Identification and isolation of differentially expressed genes from very small tissue samples. Biotechniques. 1999;26:884–892. doi: 10.2144/99265st01. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez P, Epstein DL, Borrás T. Characterization of gene expression in human trabecular meshwork using single-pass sequencing of 1060 clones. Invest Ophthalmol Vis Sci. 2000;41:3678–3693. [PubMed] [Google Scholar]
- 8.Vittitow J, Borrás T. Genes expressed in the human trabecular meshwork during pressure-induced homeostatic response. J Cell Physiol. 2004;201:126–137. doi: 10.1002/jcp.20030. [DOI] [PubMed] [Google Scholar]
- 9.Wirtz MK, Samples JR, Xu H, Severson T, Acott TS. Expression profile and genome location of cDNA clones from an infant human trabecular meshwork library. Invest Ophthalmol Vis Sci. 2002;43:3698–3704. [PubMed] [Google Scholar]
- 10.Tomarev SI, Wistow G, Raymond V, Dubois S, Malyukova I. Gene expression profile of the human trabecular meshwork: NEIBank sequence tag analysis. Invest Ophthalmol Vis Sci. 2003;44:2588–2596. doi: 10.1167/iovs.02-1099. [DOI] [PubMed] [Google Scholar]
- 11.Borrás T, Rowlette LL, Tamm ER, Gottanka J, Epstein DL. Effects of elevated intraocular pressure on outflow facility and TIGR/MYOC expression in perfused human anterior segments. Invest Ophthal-mol Vis Sci. 2002;43:33–40. [PubMed] [Google Scholar]
- 12.Price PA, Urist MR, Otawara Y. Matrix Gla protein, a new gamma-carboxyglutamic acid-containing protein which is associated with the organic matrix of bone. Biochem Biophys Res Commun. 1983;117:765–771. doi: 10.1016/0006-291x(83)91663-7. [DOI] [PubMed] [Google Scholar]
- 13.Price PA, Faus SA, Williamson MK. Warfarin-induced artery calcification is accelerated by growth and vitamin D. Arterioscler Thromb Vasc Biol. 2000;20:317–327. doi: 10.1161/01.atv.20.2.317. [DOI] [PubMed] [Google Scholar]
- 14.Wallin R, Cain D, Hutson SM, Sane DC, Loeser R. Modulation of the binding of matrix Gla protein (MGP) to bone morphogenetic protein-2 (BMP-2) Thromb Haemost. 2000;84:1039–1044. [PubMed] [Google Scholar]
- 15.Boström K, Tsao D, Shen S, Wang Y, Demer LL. Matrix GLA protein modulates differentiation induced by bone morphogenetic protein-2 in C3H10T1/2 cells. J Biol Chem. 2001;276:14044–14052. doi: 10.1074/jbc.M008103200. [DOI] [PubMed] [Google Scholar]
- 16.Ducy P, Karsenty G. The family of bone morphogenetic proteins. Kidney Int. 2000;57:2207–2214. doi: 10.1046/j.1523-1755.2000.00081.x. [DOI] [PubMed] [Google Scholar]
- 17.Luo G, Ducy P, McKee MD, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 18.Price PA, Faus SA, Williamson MK. Warfarin causes rapid calcification of the elastic lamellae in rat arteries and heart valves. Arterioscler Thromb Vasc Biol. 1998;18:1400–1407. doi: 10.1161/01.atv.18.9.1400. [DOI] [PubMed] [Google Scholar]
- 19.Hayden MR, Tyagi SC, Kolb L, Sowers JR, Khanna R. Vascular ossification-calcification in metabolic syndrome, type 2 diabetes mellitus, chronic kidney disease, and calciphylaxis-calcific uremic arteriolopathy: the emerging role of sodium thiosulfate. Cardio-vasc Diabetol. 2005;4:4. doi: 10.1186/1475-2840-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teebi AS, Lambert DM, Kaye GM, Al Fifi S, Tewfik TL, Azouz EM. Keutel syndrome: further characterization and review. Am J Med Genet. 1998;78:182–187. [PubMed] [Google Scholar]
- 21.Munroe PB, Olgunturk RO, Fryns JP, et al. Mutations in the gene encoding the human matrix Gla protein cause Keutel syndrome. Nat Genet. 1999;21:142–144. doi: 10.1038/5102. [DOI] [PubMed] [Google Scholar]
- 22.Hur DJ, Raymond GV, Kahler SG, Riegert-Johnson DL, Cohen BA, Boyadjiev SA. A novel MGP mutation in a consanguineous family: review of the clinical and molecular characteristics of Keutel syndrome. Am J Med Genet A. 2005;135:36–40. doi: 10.1002/ajmg.a.30680. [DOI] [PubMed] [Google Scholar]
- 23.Magne D, Julien M, Vinatier C, Merhi-Soussi F, Weiss P, Guicheux J. Cartilage formation in growth plate and arteries: from physiology to pathology. Bioessays. 2005;27:708–716. doi: 10.1002/bies.20254. [DOI] [PubMed] [Google Scholar]
- 24.Mathieu P, Voisine P, Pepin A, Shetty R, Savard N, Dagenais F. Calcification of human valve interstitial cells is dependent on alkaline phosphatase activity. J Heart Valve Dis. 2005;14:353–357. [PubMed] [Google Scholar]
- 25.Johnson DH, Tschumper RC. Human trabecular meshwork organ culture: a new method. Invest Ophthalmol Vis Sci. 1987;28:945–953. [PubMed] [Google Scholar]
- 26.Borrás T, Tamm ER, Zigler JS., Jr Ocular adenovirus gene transfer varies in efficiency and inflammatory response. Invest Ophthalmol Vis Sci. 1996;37:1282–1293. [PubMed] [Google Scholar]
- 27.Zigler JS, Jr, Hess HH. Cataracts in the Royal College of Surgeons rat: evidence for initiation by lipid peroxidation products. Exp Eye Res. 1985;41:67–76. doi: 10.1016/0014-4835(85)90095-8. [DOI] [PubMed] [Google Scholar]
- 28.Borrás T, Rowlette LL, Erzurum SC, Epstein DL. Adenoviral reporter gene transfer to the human trabecular meshwork does not alter aqueous humor outflow: relevance for potential gene therapy of glaucoma. Gene Ther. 1999;6:515–524. doi: 10.1038/sj.gt.3300860. [DOI] [PubMed] [Google Scholar]
- 29.Olmsted EA, Blum JS, Rill D, et al. Adenovirus-mediated BMP2 expression in human bone marrow stromal cells. J Cell Biochem. 2001;82:11–21. doi: 10.1002/jcb.1106. [DOI] [PubMed] [Google Scholar]
- 30.Sweatt A, Sane DC, Hutson SM, Wallin R. Matrix Gla protein (MGP) and bone morphogenetic protein-2 in aortic calcified lesions of aging rats. J Thromb Haemost. 2003;1:178–185. doi: 10.1046/j.1538-7836.2003.00023.x. [DOI] [PubMed] [Google Scholar]
- 31.Wallin R, Sane DC, Hutson SM. Vitamin K 2,3-epoxide reductase and the vitamin K-dependent gamma-carboxylation system. Thromb Res. 2002;108:221–226. doi: 10.1016/s0049-3848(03)00060-4. [DOI] [PubMed] [Google Scholar]
- 32.Zebboudj AF, Shin V, Boström K. Matrix GLA protein and BMP-2 regulate osteoinduction in calcifying vascular cells. J Cell Biochem. 2003;90:756–765. doi: 10.1002/jcb.10669. [DOI] [PubMed] [Google Scholar]
- 33.Wallin R, Wajih N, Greenwood GT, Sane DC. Arterial calcification: a review of mechanisms, animal models, and the prospects for therapy. Med Res Rev. 2001;21:274–301. doi: 10.1002/med.1010. [DOI] [PubMed] [Google Scholar]
- 34.Proudfoot D, Skepper JN, Shanahan CM, Weissberg PL. Calcification of human vascular cells in vitro is correlated with high levels of matrix Gla protein and low levels of osteopontin expression. Arterioscler Thromb Vasc Biol. 1998;18:379–388. doi: 10.1161/01.atv.18.3.379. [DOI] [PubMed] [Google Scholar]
- 35.Shin V, Zebboudj AF, Bostrom K. Endothelial cells modulate osteogenesis in calcifying vascular cells. J Vasc Res. 2004;41:193–201. doi: 10.1159/000077394. [DOI] [PubMed] [Google Scholar]
- 36.Wordinger RJ, Agarwal R, Talati M, Fuller J, Lambert W, Clark AF. Expression of bone morphogenetic proteins (BMP), BMP receptors, and BMP associated proteins in human trabecular meshwork and optic nerve head cells and tissues. Mol Vis. 2002;8:241–250. [PubMed] [Google Scholar]
- 37.Chow RL, Lang RA. Early eye development in vertebrates. Annu Rev Cell Dev Biol. 2001;17:255–296. doi: 10.1146/annurev.cellbio.17.1.255. [DOI] [PubMed] [Google Scholar]
- 38.Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev. 1995;9:2808–2820. doi: 10.1101/gad.9.22.2808. [DOI] [PubMed] [Google Scholar]
- 39.Zhao S, Chen Q, Hung FC, Overbeek PA. BMP signaling is required for development of the ciliary body. Development. 2002;129:4435–4442. doi: 10.1242/dev.129.19.4435. [DOI] [PubMed] [Google Scholar]
- 40.Chang B, Smith RS, Peters M, et al. Haploinsufficient Bmp4 ocular phenotypes include anterior segment dysgenesis with elevated intraocular pressure. BMC Genet. 2001;2:18. doi: 10.1186/1471-2156-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li J, Tripathi BJ, Chalam KV, Tripathi RC. Transforming growth factor-beta 1 and -beta 2 positively regulate TGF-beta 1 mRNA expression in trabecular cells. Invest Ophthalmol Vis Sci. 1996;37:2778–2782. [PubMed] [Google Scholar]
- 42.Tripathi RC, Li J, Chan WF, Tripathi BJ. Aqueous humor in glaucomatous eyes contains an increased level of TGF-beta 2. Exp Eye Res. 1994;59:723–727. doi: 10.1006/exer.1994.1158. [DOI] [PubMed] [Google Scholar]
- 43.Boström K, Zebboudj AF, Yao Y, Lin TS, Torres A. Matrix GLA protein stimulates VEGF expression through increased transforming growth factor-beta1 activity in endothelial cells. J Biol Chem. 2004;279:52904–52913. doi: 10.1074/jbc.M406868200. [DOI] [PubMed] [Google Scholar]
- 44.Azhar M, Schultz JJ, Grupp I, et al. Transforming growth factor beta in cardiovascular development and function. Cytokine Growth Factor Rev. 2003;14:391–407. doi: 10.1016/s1359-6101(03)00044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


