Figure 1.
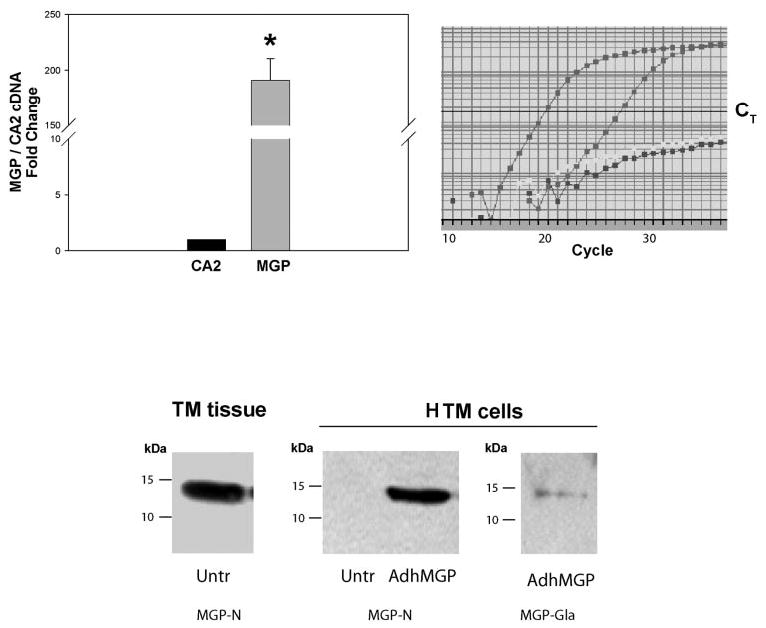
Characterization of MGP mRNA and protein in human intact TM tissue and HTM cells. Top: Relative MGP mRNA abundance. RNA from the pooled TMs from perfused anterior segments of an 82-year-old white donor, was reversed transcribed and identical aliquots amplified with specific probe-primers for MGP (Hs00179899_m1) and CA2 (Hs00163869_m1) (real-time PCR). The expression of MGP was compared with that of CA2 and expressed as x-fold change ± SEM. Right: a representative graph from one reaction (left curve: MGP; middle curve: CA2; right curve: nontemplate control for MGP and CA2). *P ≤ 0.00002, n = 4. MGP cDNA abundance was significantly higher than that of CA2. Bottom: MGP protein detection by Western blot analysis. Insoluble proteins extracted from a single TM from perfused anterior segment (77-year-old white donor; left) and HTM cells (right) either untreated or infected with recombinant adenovirus AdhMGP (650 VP per cell). Proteins were separated on 15% SDS-polyacrylamide gels, transferred to PVDF membranes and incubated with rabbit polyclonal MGP antibodies.30 MGP-N is an N-terminal peptide antibody that recognizes total MGP. MGP GLA is a GLA-peptide antibody that specifically recognizes fully γ-carboxylated MGP. MGP was detected in perfused intact TM tissue but not in untreated HTM cells. Both, total MGP and carboxylated MGP were found in AdhMGP-infected HTM cells.
