Figure 3.
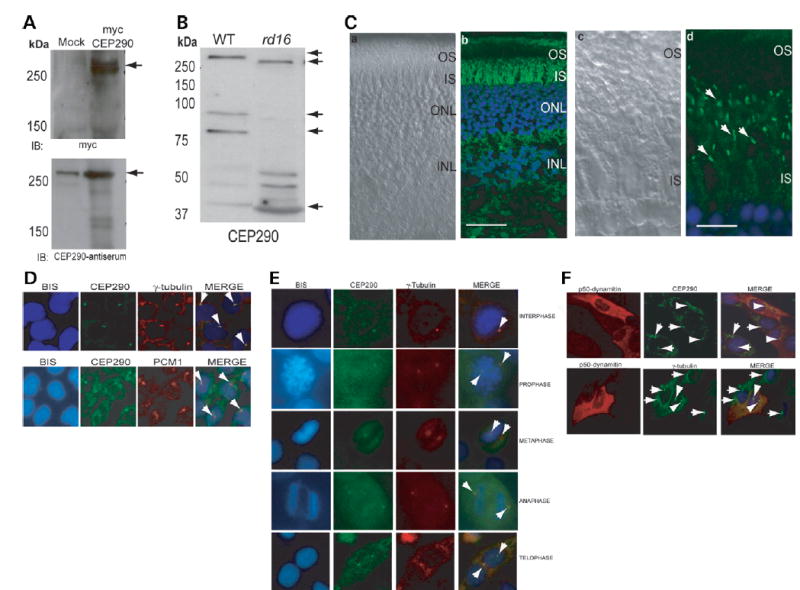
Expression and localization of CEP290. (A) COS-1 cells were transfected with empty vector (mock) or a vector encoding full-length human CEP290 protein fused to a myc-tag. Cells were lysed and analyzed by immunoblotting (IB), using anti-myc (upper panel) or anti-CEP290 antiserum (lower panel). Arrows indicate specific bands. The immunoreactive band in the mock transfected lane (lower panel) is endogenous CEP290 protein. Pre-immune serum shows no signal (data not shown). (B) Immunoblots of protein extracts from WT (20 μg) and rd16 (200μg) retina were analyzed using CEP290 antibody. Arrows indicate the full length and predicted alternatively spliced products of CEP290. (C) Immunohistochemical analysis of WT mouse retina. The sections were incubated with the CEP290 antibody followed by secondary antibody incubation. (a) and (c) Nomarski image of the retinal sections. (b) and (d) Staining with the CEP290 antibody (green) reveals intense labeling of the connecting cilium (indicated by arrows). Labeling in the IS is also observed. Scale bar: 40 μm for (a), (b); 10 μm for (c), (d). (D) CEP290 (green) co-localizes with γ-tubulin (red; upper panel) and PCM1 (red; lower panel) at the centrosomes (arrows; merge) in IMCD-3 cells. Bisbenzimide (BIS) was used to stain the DNA. (E) CEP290 is associated with centrosomes during cell cycle. Synchronized HeLa cells were co-stained with antibodies against γ-tubulin (red) and CEP290 (green) and analyzed by confocal microscopy. Arrows indicate the centrosomal staining of CEP290 (merge) at all indicated stages of cell division. (F) IMCD-3 cells were transfected with p50-dynamitin expression construct. Cells were stained with p50 (red), CEP290 or γ-tubulin (green) antibodies. Arrows denote centrosomal CEP290 and γ-tubulin in untransfected cells, whereas arrowheads denote the localization of CEP290 and γ-tubulin to centrosomes in p50-overexpressing cells. Merge image shows blue nuclear staining.
