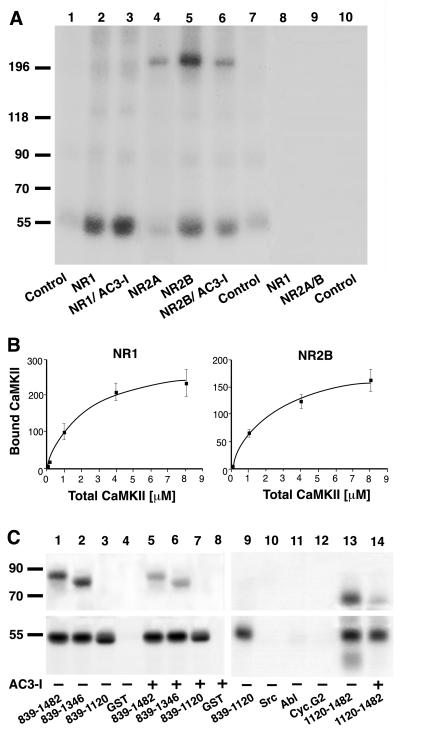
Figure 3.
CaMKIIα binding to NR1 and NR2B. (A and B) Crude membrane fractions were extracted and NMDA receptor subunits were dissociated with SDS before immunoprecipitation of individual subunits as indicated on the bottom (A) or top (B). Control immunoprecipitations were performed with nonspecific murine (A, lane 1) or rabbit IgG (A, lanes 7 and 10). Recombinant CaMKIIα was preincubated under phosphorylation conditions in the presence of [γ-32P]ATP for labeling by autophosphorylation, and immunocomplexes were incubated with the whole phosphorylation mixture. The signal at 55 kDa (A, lanes 2, 3, 5, and 6) reflects binding of recombinant and autophosphorylated CaMKIIα rather than the presence of the endogenous kinase because no phosphorylation was detectable if recombinant CaMKIIα was omitted (A, lanes 8–10). Phosphorylation of NR2B (A, lane 6) but not association of CaMKIIα with NR1 (A, lane 3) or NR2B (A, lane 6) was inhibited if the competitive catalytic site inhibitor AC3-I (20 μM) was added after autophosphorylation of CaMKIIα (27). Similar results were obtained in two other experiments. Binding of CaMKIIα to NR1 and NR2B was saturable (B; the amounts of CaMKIIα associated with NR1 or NR2B were quantified after SDS/PAGE by using PhosphorImager analysis; values are means ± SEM; n = 3). (C) CaMKIIα was preincubated under phosphorylation conditions with [γ-32P]ATP for labeling by autophosphorylation. Twenty microliters of glutathione Sepharose loaded with approximately 2 μg (as confirmed by staining with Coomassie brilliant blue) of GST or GST fusion proteins (as indicated at the bottom) then was added (together with 20 μM AC3-I when indicated) before samples were washed and analyzed by SDS/PAGE and autoradiog raphy. GST-NR2B839–1482, 839–1346, and 1120–1482, but not 839–1120 or GST alone, were phosphorylated by CaMKII in an AC3-I- sensitive manner (Upper). Autophosphorylated CaMKIIα bound equally well to all four GST-NR2B fusion proteins but not to GST alone or to nonrelevant GST fusion proteins with the Src or Abl SH3 domain or cyclin G2 (Lower).