Abstract
Protease-activated receptors (PARs) are G-protein-coupled receptors which initiate inflammatory responses when activated by specific serine proteases. This study was conducted to examine whether human conjunctival epithelial cells (HCECs) express functionally active PAR1 and PAR2 using Chang conjunctival epithelial cells as in vitro model. We performed RT-PCR and immunofluorescence analyses to determine the expression of PAR1 and PAR2, and monitored the production of IL-6 after activating HCECs with PAR1 activating agents (thrombin or TFLLRN) or PAR2 activating agents (tryptase, trypsin, or SLIGKV). The results show that HCECs constitutively express PAR1 and PAR2 mRNA and proteins, and produce significant amounts of IL-6 when incubated with specific PAR-activating enzymes or agonist peptides. Thrombin- and tryptase-induced HCEC activation was blocked by PAR1 and PAR2 neutralizing antibodies, respectively, and by specific enzyme inhibitors. The constitutive expression of PAR1 and PAR2, and their activation by thrombin and tryptase, respectively, may have important implications in ocular inflammation.
INTRODUCTION
Allergic inflammation of the ocular conjunctiva is associated with increased mast cell mediators in tear fluid, and the recruitment of activated eosinophils, mast cells, and lymphocytes [1]. Furthermore, ocular epithelial cells are active participants in the regulation of allergic inflammation via expression of adhesion molecules and elaboration of proinflammatory cytokines and chemokines [2]. Recent work has highlighted the potential role of protease-activated receptors (PARs) in stimulating cytokine production by respiratory epithelium [3]. In addition, Lang et al [4] have demonstrated the presence of PAR1 and PAR2 in the corneal epithelium. However, the expression and functions of PAR1 and PAR2 in bulbar conjunctival epithelium have not been explored.
PARs are G-protein-coupled seven transmembrane receptors [5, 6] with a unique signaling mechanism. These receptors have their own ligands embedded in their extracellular N-terminal domains. Cleavage of the extracellular domain by a specific serine protease frees the tethered ligand to activate the receptor [5]. Initial reports demonstrated that this mechanism was operative in thrombin-induced platelet activation [6]. To date, four subtypes of PARs have been described [6–9], and among these, PAR1 and PAR2 are widely expressed in many cell types including endothelial cells, platelets, bronchial epithelial cells, fibroblasts, mast cells, neurons, leukocytes, eosinophils, airway and vascular smooth muscle cells, keratinocytes, and renal tubular cells [3–14].
Because of the unique nature of their activation by proteases and the presence of active serine proteases in biological fluids, tissues, and allergens, PARs have been implicated in a variety of inflammatory responses. These include expression of P-selectin in endothelial cells [13], release of IL-6 by endothelial cells [14] and production of proinflammatory cytokine by bronchial epithelial cells [3], recruitment and activation of eosinophils [11, 15], and enhanced airway hyperreactivity [15]. Although PAR1 [6], PAR3 [7], and PAR4 [5] are activated by thrombin, PAR2 is activated by mast cell tryptase [16] and trypsin [9]. Thus, both thrombin and tryptase acting through PARs could perpetuate allergic inflammation in the eye. This hypothesis is further supported by the finding that tryptase levels are elevated in tear fluid after allergen challenge [1] and in vernal keratoconjunctivitis [17]. Mast cell numbers in the ocular epithelia and substantia propria are increased in a variety of atopic ocular disorders [18]. Finally, PAR2 is upregulated in the respiratory epithelia of patients with asthma [19]. The results presented in the report show that PAR1 and PAR2 mRNA and proteins are constitutively expressed in HCECs and their stimulation by thrombin and tryptase, respectively, results in the release of IL-6.
METHODS
Materials
Human conjunctival epithelial cells (HCECs, Wong Kilborne derivative of Chang epithelial cells) were obtained from American Tissue Type Culture Collection. Epithelial cell growth medium with N-2-hydroxyethylpiperazine-N′-2-ethane-sulfonic acid (HEPES), Hank's balanced salt solution (HBSS), trypsin-EDTA, and trypsin neutralizing solution (TNS) were purchased from Cambrex (San Diego, Calif). Eagles' minimum essential medium (MEM) and fetal bovine serum (low endotoxin) were purchased from Hyclone Laboratories (Logan, Utah). Hirudin, heparin, nonenzymic cell dissolution reagent, penicillin, and streptomycin were supplied by Sigma Chemical Co (St Louis, Mo). Thrombin, trypsin, tryptase, pertussis toxin, and trypsin inhibitor were purchased from Calbiochem (La Jolla, Calif). Human IL-6 ELISA kits were purchased from R & D systems (Minneapolis, Minn). PAR1 and PAR2 activating peptides (TFLLRN and SLIGKV) and inactive peptides which lacked consensus sequences but retained the same amino acid compositions (LFTNRL and ILSVKG) were synthesized by Syn Pep Corp (Dublin, Calif) and HPLC-purified at the Biotechnology Facility of Kansas University Medical Center (Kansas City, Kan). Mouse antihuman PAR1 (ATAP2) and mouse antihuman PAR2 (SAM11) were purchased from Santa Cruz (South San Francisco, Calif). Mouse antihuman PAR1 (WEDE 15) was purchased from Fisher Scientific. Mouse isotype IgG2a and CYTM3-conjugated affinipure goat antimouse IgG were obtained from Chemicon (Temecula, Calif) and Jackson ImmunoResearch Laboratories (West Grove, Pa), respectively. The chamber slides used for immunofluorescece studies were products of Labtec, Nalgenunc International (Naperville, Ill). Primers and RT-PCR reagents for PAR1 and PAR2 were purchased from Invitrogen (Carlsbad, Calif). Antifade was supplied by Molecular Probe (Eugene, Ore).
Culture of human conjunctival epithelial cells
HCECs were grown in Eagle's MEM with glutamine supplemented with 15 mM HEPES, 100 units/mL penicillin, 100 μg/mL streptomycin, and with or without 10% fetal bovine serum (complete medium). At confluence, the cells were detached from the culture flasks using trypsin-EDTA or cell dissolution solution (depending on the experimental conditions), washed twice, and resuspended in serum-free medium. All experiments were performed using HCECs maintained between three and nine passages after obtaining the cells from ATCC.
Assay of IL-6 production
HCECs (2 × 104) in complete medium were seeded on to each of the wells of a 96-well microtiter plate and allowed to adhere for 24 hours. Following adherence, the culture medium was removed, serum-free medium (complete medium without serum) was added, and the monolayers were incubated with selected concentrations of the agonists in a final volume of 0.2 mL serum-free medium. All incubations were carried out at 37°C in 5% humidified CO2 for 24 hours. After incubation, culture supernatants were collected and IL-6 levels quantified by ELISA according to the manufacturer's protocol.
Semiquantitative reverse transcription-polymerase chain reaction (RT-PCR) for detection of mRNA for PAR1 and PAR2
Total RNA was isolated from HCECs using TRIzol reagent and treated with RNAse–free DNAse I. For the reverse transcription reaction, superscript II RNase H − reverse transcriptase system was employed. PCR amplification was performed with Taq polymerase for 32 cycles at 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s, and, finally 72°C for 5 min. After amplification, the products were subjected to electrophoresis on a 2% agarose gel containing ethidium bromide and analyzed under UV light against DNA molecular markers. The following primers were used:
| PAR1: 5′-TGTGAACTGATCATGTTTATG-3′ and 5′-TTCGTAAGATAAGAGATATGT-3′ |
| PAR2: 5′-TGGATGAGTTTTCTGCATCTGTCC-3′ and 5′-CGTGATGTTCAGGGCAGGAATG-3′ |
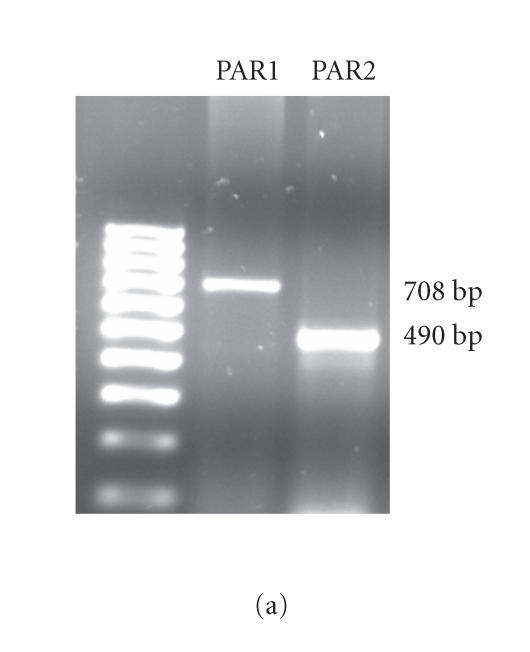
Amplification of cDNA yielded 708 and 490 base-pair products for PAR1 and PAR2, respectively, as predicted.
Determination of PAR1 and PAR2 protein expression by immunoflourescence
HCECs (2 × 104) were plated in the wells of an 8-chamber slide and incubated under the atmosphere of 5% CO2. Following adherence, cells were washed twice with phosphate buffer saline (PBS), and fixed with 4% paraformaldehyde. After fixing, cells were treated with blocking buffer (1% bovine serum albumin and 5% normal goat serum in PBS, pH 7.5, with 0.3% Triton X–100) for one hour. Primary mouse antibodies against PAR1 and PAR2 diluted 1 : 200 with the antibody diluent (1% bovine serum albumin, 0.05% sodium azide, and 5% normal goat serum in PBS, pH 7.5, with 0.3% Triton X–100) were then added. Following overnight incubation at 4°C, monolayers were washed, and treated with a secondary goat antimouse antibody (CY3) (diluted 1 : 200 with antibody diluent) for 1 hour. The monolayers were then washed with PBS, treated with antifade, and viewed with a fluorescent microscope.
statistical analysis
Statistics were computed using SPSS 10.0 (SPSS, Chicago, Ill). Two-tailed P-values < .05 were taken to signify significance. For each experiment, treatments were first compared to the medium-only control using Dunnett's test. Then, to test for differences among treatments, if the one-way ANOVA was significant, post hoc comparisons were made using the Student-Newman-Keuls test. Dose-response curves were modeled using one-df linear and quadratic orthogonal polynomial contrasts, with strength of association indexed by η2. A perfect correlation is equal to 1.
RESULTS
PAR1 and PAR2 expression in human conjunctival epithelial cells
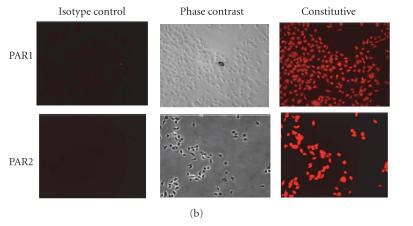
RT-PCR was used to examine PAR1 and PAR2 mRNA expression in HCECs. As shown in Figure 1(a), HCECs constitutively express PAR1 and PAR2 mRNA. Since mRNA expression does not always correlate with protein expression, immunofluorescence analyses were carried out to identify the presence of PAR1 and PAR2 proteins in HCECs. As shown in Figure 1(b), immunofluorescent staining utilizing specific antibodies confirmed that HCECs constitutively express PAR1 and PAR2 proteins.
Figure 1.
(a) RT-PCR analyses of the constitutive expression of PAR1 and PAR2 mRNA. Total RNA was extracted, reverse transcribed, and amplified using PAR1- and PAR2-specific primers as described under “Methods” section. The amplified products were electrophoresed on a 2% agarose gel. The result presented is representative of three independent experiments. (b) Immunoflourescence analyses of the expression of PAR1 and PAR2 proteins. The cultured HCECs were fixed with paraformaldehyde and were incubated with specific primary antibodies or isotype IgG. After extensive washing, the cells were incubated with rhodamine-conjugated secondary antibodies and viewed under a fluorescence microscope for imaging.
Functional activity of PAR1 and PAR2
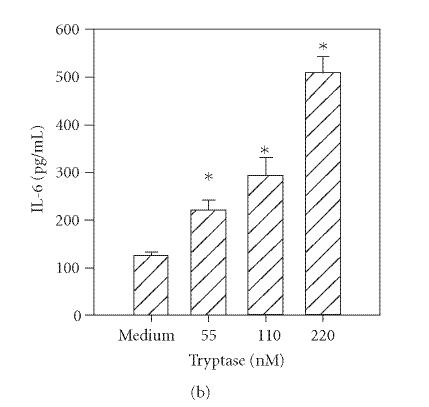
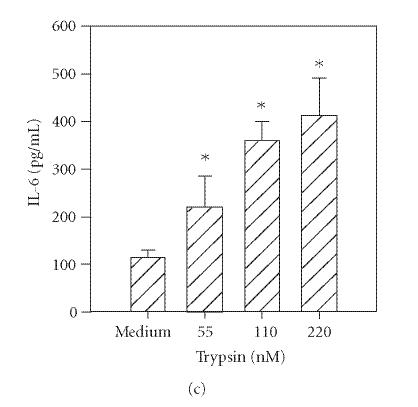
Previous reports document that thrombin induces IL-6 production by human umbilical vein endothelial cells [14] and bronchial epithelial cells [3]. In addition, trypsin induces cytokine production in corneal [4] and bronchial epithelial cells [3]. Therefore, to determine the functional responsiveness of PAR1 and PAR2, the cells were incubated with different concentrations of thrombin, tryptase, or trypsin, and the levels of secreted IL-6 were quantified by ELISA. As shown in Figure 2(a), incubation with thrombin (14–220 nM) resulted in a dose-dependent increase in IL-6 production. Incubation of HCECs with tryptase (55−220 nM) or trypsin (55−220 nM) also stimulated IL-6 production in a concentration-dependent manner (Figures 2(b) and 2(c)).
Figure 2.
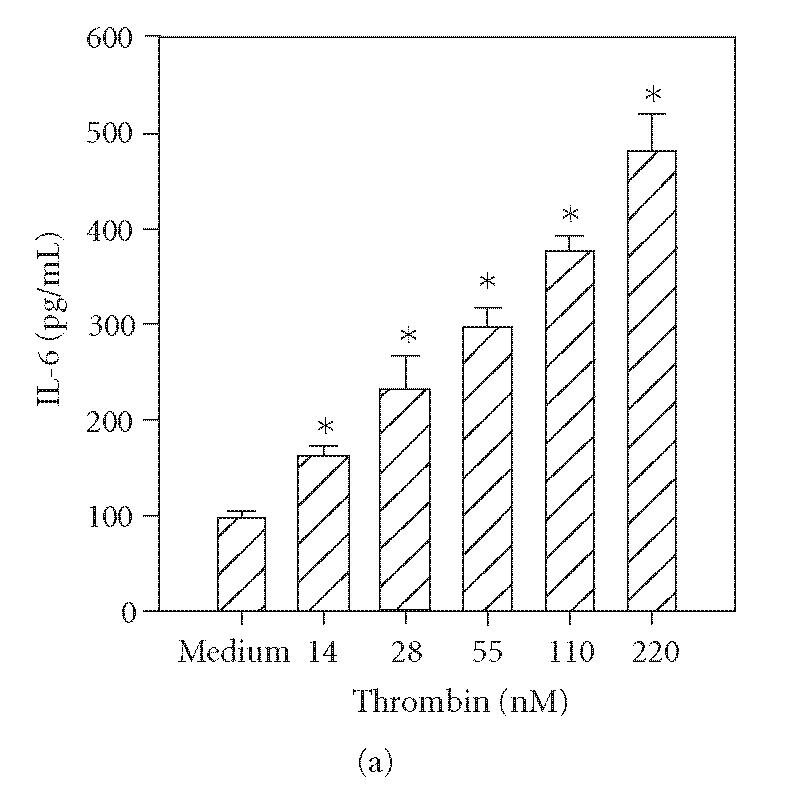
Thrombin-, tryptase-, and trypsin-stimulated IL-6 production by HCECs. HCEC monolayers were incubated with the indicated concentrations of thrombin (a), tryptase (b), or trypsin (c). After a 24-hour culture, IL-6 levels in the culture media were assayed by ELISA. Each value presented is the mean + / − SD of quadruplicate determinations. The results presented are representatives of four independent experiments.
*indicates P < .05 when compared to medium control.
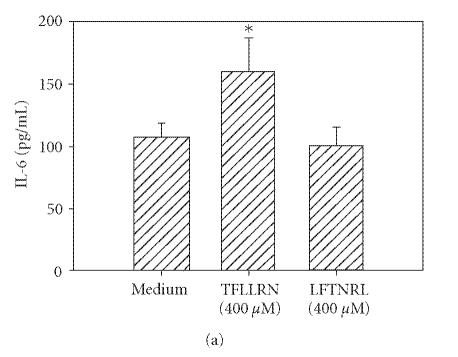
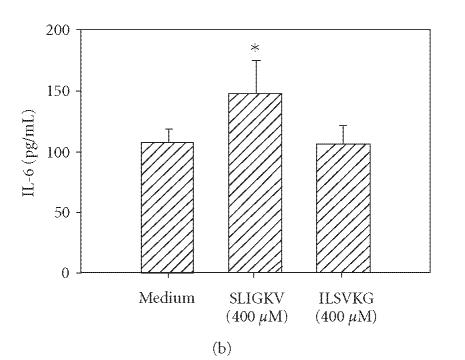
Thrombin cleavage of PAR1 exposes a new N-terminus with the sequence SFLLRN which acts as a tethered ligand. Similarly, PAR2 cleaving enzymes expose the unique sequence SLIGKV on the extracellular domain of the receptor. Therefore, synthetic hexapeptides bearing these amino acid sequences were utilized to verify the specificity of each of these PARs. These synthetic PAR-activating peptides can activate specific PARs without inducing proteolytic cleavage [10]. In the case of PAR1 agonist, we used TFLLRN instead of SFLLRN since substitution of threonine for serine prevents cross-desensitization of PAR2 and makes the agonist more PAR1-specific [20]. Incubation of HCECs with TFLLRN (Figure 3(a)) and SLIGKV (Figure 3(b)) resulted in an increase in IL-6 production. The control peptides, which lacked consensus sequences (LFTNRL and ILSKVG) but had the same amino acid composition as active peptides, did not activate HCECs to secrete IL-6.
Figure 3.
PAR1 and PAR2 agonist peptides stimulate IL-6 production in HCECs. HCEC monolayers were incubated with PAR1 agonist peptide (TFLLRN) or scrambled peptide (LFTNRL) (a), or with the PAR2 agonist peptide (SLIGKV) or inactive peptide (ILSVKG) (b). After 24 hours, IL-6 levels in the culture media were assayed by ELISA. Each value presented is the mean + / − SD of quadruplicate determinations. The results presented are representatives of six independent experiments.
*indicates P < .05 when compared to medium control.
Effects of inhibiting protease activity and G-protein coupling on PAR-mediated IL-6 release
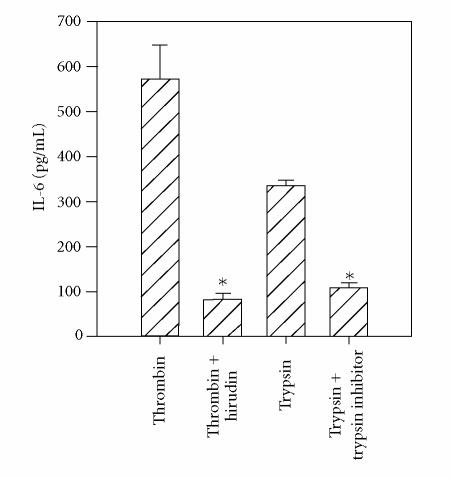
The requirement for catalytic activity for thrombin and trypsin for stimulation of PAR1 and PAR2, respectively, was confirmed by incubating thrombin with a selective inhibitor, hirudin, and trypsin with a selective inhibitor, soybean trypsin inhibitor. As depicted in Figure 4, the ability of thrombin and trypsin to induce IL-6 production by HCECs was completely abrogated when enzymes were treated with their specific inhibitors. In control studies, hirudin did not inhibit trypsin activity nor did soybean trypsin inhibitor inhibit thrombin activity on HCECs (data not shown).
Figure 4.
Requirement of the enzymic activity of thrombin and trypsin for HCEC activation. HCEC monolayers were incubated with thrombin (220 nM), thrombin + hirudin (4 U/mL), trypsin, or trypsin + soybean trypsin inhibitor (STI) (100 U/mL) for 24 hours at 37°C. Unactivated cells produced 107 ± 12 pg/mL of IL-6. The presence of hirudin or STI to unstimulated cells did not alter IL-6 production. The results presented are representatives of two independent experiments.
*indicates P < .05 when compared to medium control.
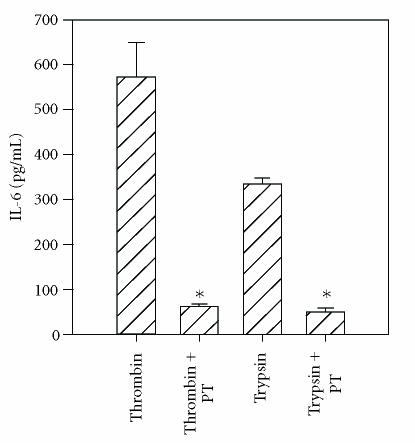
It is well-recognized that the PAR-signaling pathway requires G-protein coupling [5, 21]. Therefore, the effect of the G-protein-coupled receptor inhibitor, pertussis toxin, on thrombin- and tryptase-induced IL-6 production was tested. As shown in Figure 5, preincubation of HCECs with pertussis toxin for 30 minutes prior to the addition of thrombin or trypsin completely inhibited IL-6 release.
Figure 5.
Inhibition of thrombin- and trypsin-induced IL-6 production by pertussis toxin. The HCEC monolayers were incubated with pertussis toxin (PT) (1 μg/mL) for 30 minutes and challenged with thrombin (220 nM) or trypsin (220 nM). Unactivated cells produced 107 ± 12 pg/mL of IL-6. The results presented are representative of two independent experiments.
*indicates P < .05 when compared to medium control.
Inhibition of PAR1 and PAR2 activation by receptor specific antibody
To further determine the specificity of PAR-mediated activation, HCECs were incubated with specific antibodies that block the enzymatic cleavage of PARs. The antibodies WEDE 15 and ATAP 2 bind PAR1 near its cleavage site. This combination has previously been shown to result in complete inhibition of thrombin-induced activation of human umbilical endothelial cells [24]. The antibody SAM11 binds to PAR2 near its cleavage region. As shown in Figure 6(a), incubation of HCECs with thrombin in the presence of WEDE 15 plus ATAP 2 resulted in the complete inhibition of thrombin-stimulated IL-6 production, whereas PAR2 neutralizing antibodies showed no effect (Figure 6(a)). Similarly, incubation of HCECs with PAR2 blocking antibody SAM11 resulted in complete inhibition of trypsin- and tryptase- induced IL-6 production (Figures 6(b) and 6(c)), while antibodies against PAR1 were without effect.
Figure 6.
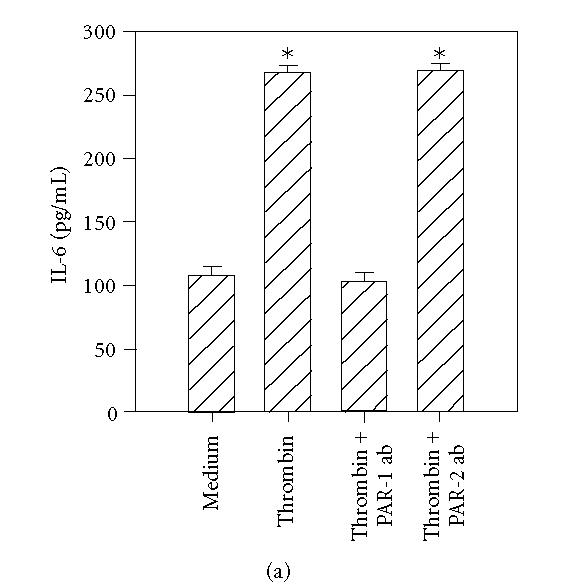
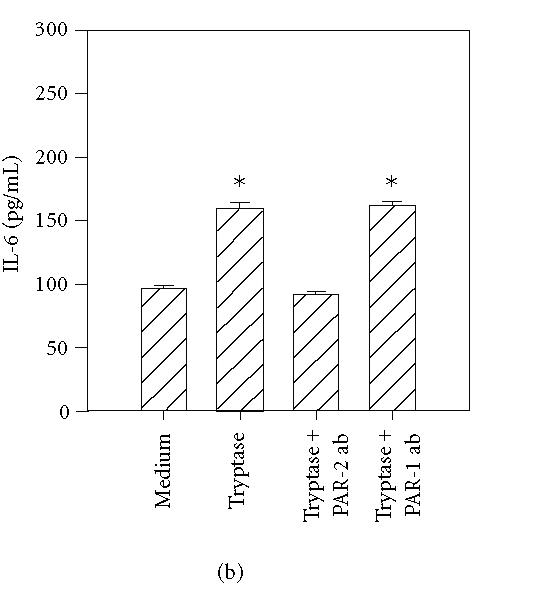
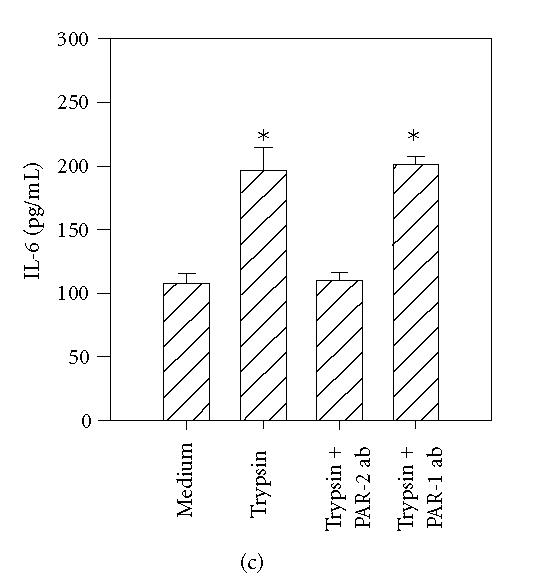
PAR1 and PAR2 neutralizing antibodies selectively inhibit the effects of thrombin, tryptase, and trypsin. HCEC monolayers were incubated for 15 minutes with a cocktail of PAR1 blocking antibodies, ATAP-2 (25 μg/mL), and WEDE 15 (25 μg/mL), or the PAR2 blocking antibody, SAM11 (25 μg/mL), prior to stimulation for 24 hours with thrombin (50 nM) (a), tryptase (50 nM) (b), and trypsin (50 nM) (c). IL-6 levels in the culture media were then assayed by ELISA. Each value presented is the mean + / − SD of quadruplicate determinations.
*indicates P < .05 when compared to medium control.
DISCUSSION
The results of the present study demonstrate for the first time that HCECs constitutively express PAR1 and PAR2 mRNA and protein (Figures 1(a) and 1(b)). Furthermore, PAR1 and PAR2 specific proteases induce a significant increase in IL-6 production (Figure 2). This response can be blocked with the addition of specific antibodies that bind near the cleavage site of PAR1 or PAR2 or with selective inhibitors of the protease. Finally, the specificity of the PAR1 and PAR2 mediated response is confirmed by the ability of the PAR1 activating peptide TFLLRN and the PAR2 activating peptide SLIGKV to induce IL-6 production by HCECs. This confirms that both PARs are expressed and are functional.
It has been shown previously that both trypsin and tryptase at high concentrations may cleave PAR1 in vitro, in addition to PAR2 [16]. In the present study, incubation of HCECs with the antibodies to the PAR1 cleavage region did not inhibit the effects of trypsin and tryptase, whereas PAR2 blocking antibodies completely abrogated the effects of these enzymes. This indicates that the effects of thrombin, trypsin, and tryptase are indeed mediated through specific activation of PAR1 and PAR2, and there was no crosstalk between the PARs at the concentrations of the proteases used here. These results are consistent with a previous report that demonstrated the cleavage of peptides derived from PAR1 with high levels of trypsin and tryptase in transfected COS-1 cells, but no cleavage of endogenous PAR1 in platelets or CHRF-288 cells [16]. Alternatively, since thrombin has been reported to cleave PAR3 and PAR4 [5, 21], cell activation could be attributed to these receptors, if present. However, the complete inhibition of activation attained with the PAR1 antibodies suggests that the thrombin effect in this cell line is due to PAR1.
The activation of PAR1 and PAR2 shares common signal transduction pathways including G-protein-coupled receptor activation [5, 21]. The complete inhibition of thrombin- and tryptase-induced IL-6 release by pertussis toxin (Figure 5) confirms the involvement of G-proteins in protease-mediated HCEC activation. One could speculate that PAR2, present in ocular conjunctiva and known to be upregulated in allergic airways disease, is activated by tryptase released as a result of mast cell degranulation. Mast cell tryptase is increased in tear fluid in a variety of allergic ocular diseases [17, 22, 23]. The protease activity of tear fluid tryptase, however, is not known, but should be studied to ascertain if the above mechanisms are plausible. Another recently described activator of PAR2 in the airway is human airway trypsin-like protease (HAT), which has been shown to activate PAR2 in human bronchial epithelial cells [25] and in psoriatic skin [26]. The presence of an additional PAR2 agonist in tear fluid is unknown. The presence of an endogenous PAR1 activator in the ocular conjunctiva has not been identified. Although recent work has pointed to increased thrombin activity in human airway BAL fluid in an allergen challenge model [27], thrombin activity in tear fluid has not been reported. Future studies are needed to characterize protease activity in tear fluid and determine the relevant physiology of PAR receptors in the conjunctiva. In the current study with the HCECs, the proteases including thrombin, trypsin, and tryptase are equally active in stimulating IL-6 release when compared on a molar basis.
Of particular relevance to the role of PARs in conjunctival inflammation is the finding that some allergens with protease activity have the potential to directly activate PARs. King et al [28] showed that the mite cysteine and serine proteolytic allergens, Der p-1 and Der p-9, induced cytokine production from human bronchial epithelial cells. This effect could be blocked with protease inhibitors. Further work has demonstrated that Der p-1 [29], Der p-3 [30], and Der p-9 [30] activation of respiratory epithelium is mediated, at least partially, by PAR2. More recently, cockroach extract has been shown to activate PAR2 in human airway epithelial cells [31, 32], and the cysteine proteases, papain, and Der f-1 can activate eosinophils [33, 34]. If proteolytic allergen activation of conjunctival PARs also occurs, these receptors may play a role in inflammation independent of allergic sensitization and mast cell degranulation. This is particularly relevant in the eye which, along with the respiratory epithelium, represents a major site of environmental allergen exposure. Whether this phenomenon may cause symptoms irrespective of IgE sensitization, or whether this may simply facilitate or augment initial sensitization by inducing an early inflammatory infiltrate, is currently unknown.
Numerous reports also show that PARs may play important roles in allergic airway disease [35–39]. In murine models, PAR knockout mice have shown marked attenuation in eosinophil recruitment and airway hyperreactivity in response to allergen challenge [40]. The presence of PAR1 and PAR2 in the conjunctival and corneal epithelium provides the opportunity to uncover their physiologic relevance and develop strategies using peptide agonists and/or antagonists to manipulate disease processes at the ocular surface.
ACKNOWLEDGMENTS
We thank John Belmont in the Department of Pediatrics for his help with the statistical analysis. This study was supported by Grants from the Joseph and Elizabeth Carey Arthritis Fund, Lied Foundation, and the Kansas University Endowment Association. Both T. J. Nickel and M. H. Kabir contributed equally to this work.
ABBREVIATIONS
ABBREVIATIONS
- PAR:
Protease-activated receptor.
- HCECs:
Human conjunctival epithelial cells.
- PAR-AP:
Protease-activated receptor activating peptide.
- TFLLRN:
PAR1 activating peptide, re-phe-leu-leu-arg-asn.
- SLIGKV:
PAR2 activating peptide, ser-leu-iso-gly-lys-val.
- SBTI:
Soybean trypsin inhibitor.
References
- 1.Bacon AS, Ahluwalia P, Irani AM, et al. Tear and conjunctival changes during the allergen-induced early- and late-phase responses. Journal of Allergy and Clinical Immunology. 2000;106(5):948–954. doi: 10.1067/mai.2000.110930. [DOI] [PubMed] [Google Scholar]
- 2.Irkec M, Bozkurt B. Epithelial cells in ocular allergy. Current Allergy and Asthma Reports. 2003;3(4):352–357. doi: 10.1007/s11882-003-0098-2. [DOI] [PubMed] [Google Scholar]
- 3.Asokananthan N, Graham PT, Fink J, et al. Activation of protease-activated receptor (PAR)-1, PAR-2, and PAR-4 stimulates IL-6, IL-8, and prostaglandin E2 release from human respiratory epithelial cells. Journal of Immunology. 2002;168(7):3577–3585. doi: 10.4049/jimmunol.168.7.3577. [DOI] [PubMed] [Google Scholar]
- 4.Lang R, Song PI, Legat FJ, et al. Human corneal epithelial cells express functional PAR-1 and PAR-2. Investigative Ophthalmology and Visual Science. 2003;44(1):99–105. doi: 10.1167/iovs.02-0357. [DOI] [PubMed] [Google Scholar]
- 5.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407(6801):258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 6.Vu TK, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64(6):1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- 7.Ishihara H, Connolly AJ, Zeng D, et al. Protease-activated receptor 3 is a second thrombin receptor in humans. Nature. 1997;386(6624):502–506. doi: 10.1038/386502a0. [DOI] [PubMed] [Google Scholar]
- 8.Xu WF, Andersen H, Whitmore TE, et al. Cloning and characterization of human protease-activated receptor 4. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(12):6642–6646. doi: 10.1073/pnas.95.12.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nystedt S, Emilsson K, Wahlestedt C, Sundelin J. Molecular cloning of a potential proteinase activated receptor. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(20):9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hollenberg M, Compton S. Proteinase-activated receptors. Pharmacological Reviews. 2002;54:203–217. doi: 10.1124/pr.54.2.203. [DOI] [PubMed] [Google Scholar]
- 11.Miike S, McWilliam AS, Kita H. Trypsin induces activation and inflammatory mediator release from human eosinophils through protease-activated receptor-2. Journal of Immunology. 2001;167(11):6615–6622. doi: 10.4049/jimmunol.167.11.6615. [DOI] [PubMed] [Google Scholar]
- 12.D'Andrea MR, Derian CK, Leturcq D, et al. Characterization of protease-activated receptor-2 immunoreactivity in normal human tissues. Journal of Histochemistry and Cytochemistry. 1998;46(2):157–164. doi: 10.1177/002215549804600204. [DOI] [PubMed] [Google Scholar]
- 13.Lindner J, Kahn M, Coughlin S, et al. Delayed onset of inflammation in protease-activated receptor-2-deficient mice. Journal of Immunology. 2000;165(11):6504–6510. doi: 10.4049/jimmunol.165.11.6504. [DOI] [PubMed] [Google Scholar]
- 14.Chi L, Li Y, Stehno-Bittel L, et al. Interleukin-6 production by endothelial cells via stimulation of protease-activated receptors is amplified by endotoxin and tumor necrosis factor-α. Journal of Interferon and Cytokine Research. 2001;21(4):231–240. doi: 10.1089/107999001750169871. [DOI] [PubMed] [Google Scholar]
- 15.Schmidlin F, Amadesi S, Vidil R, et al. Expression and function of proteinase-activated receptor 2 in human bronchial smooth muscle. American Journal of Respiratory and Critical Care Medicine. 2001;164(7):1276–1281. doi: 10.1164/ajrccm.164.7.2101157. [DOI] [PubMed] [Google Scholar]
- 16.Molino M, Barnathan ES, Numerof R, et al. Interactions of mast cell tryptase with thrombin receptors and PAR-2. Journal of Biological Chemistry. 1997;272(7):4043–4049. doi: 10.1074/jbc.272.7.4043. [DOI] [PubMed] [Google Scholar]
- 17.Tabbara KF. Tear tryptase in vernal keratoconjunctivitis. Archives of Ophthalmology. 2001;119(3):338–342. doi: 10.1001/archopht.119.3.338. [DOI] [PubMed] [Google Scholar]
- 18.Baddeley SM, Bacon AS, McGill JI, Lightman SL, Holgate ST, Roche WR. Mast cell distribution and neutral protease expression in acute and chronic allergic conjunctivitis. Clinical and Experimental Allergy. 1995;25(1):41–50. doi: 10.1111/j.1365-2222.1995.tb01001.x. [DOI] [PubMed] [Google Scholar]
- 19.Knight DA, Lim S, Scaffidi AK, et al. Protease-activated receptors in human airways: upregulation of PAR-2 in respiratory epithelium from patients with asthma. Journal of Allergy and Clinical Immunology. 2001;108(5):797–803. doi: 10.1067/mai.2001.119025. [DOI] [PubMed] [Google Scholar]
- 20.Hollenberg MD, Saifeddine M, Al-Ani B, Kawabata A. Proteinase-activated receptors: structural requirements for activity, receptor cross-reactivity, and receptor selectivity of receptor-activating peptides. Canadian Journal of Physiology and Pharmacology. 1997;75(7):832–841. [PubMed] [Google Scholar]
- 21.Ossovskaya VS, Bunnett NW. Protease-activated receptors: contribution to physiology and disease. Physiological Reviews. 2004;84(2):579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- 22.Butrus SI, Ochsner KI, Abelson MB, Schwartz LB. The level of tryptase in human tears. An indicator of activation of conjunctival mast cells. Ophthalmology. 1990;97(12):1678–1683. doi: 10.1016/s0161-6420(90)32362-x. [DOI] [PubMed] [Google Scholar]
- 23.Magrini L, Bonini S, Centofanti M, Schiavone M, Bonini S. Tear tryptase levels and allergic conjunctivitis. Allergy. 1996;51(8):577–581. doi: 10.1111/j.1398-9995.1996.tb04671.x. [DOI] [PubMed] [Google Scholar]
- 24.O'Brien P, Prevost N, Molino M, et al. Thrombin responses in human endothelial cells. The Journal of Biological Chemistry. 2000;275(18):13502–13509. doi: 10.1074/jbc.275.18.13502. [DOI] [PubMed] [Google Scholar]
- 25.Miki M, Nakamura Y, Takahashi A, et al. Effect of human airway trypsin-like protease on intracellular free Ca2+ concentration in human bronchial epithelial cells. Journal of Medical Investigation. 2003;50(1-2):95–107. [PubMed] [Google Scholar]
- 26.Iwakiri K, Ghazizadeh M, Jin E, et al. Human airway trypsin-like protease induces PAR-2-mediated IL-8 release in psoriasis vulgaris. The Journal of Investigative Dermatology. 2004;122(4):937–944. doi: 10.1111/j.0022-202X.2004.22415.x. [DOI] [PubMed] [Google Scholar]
- 27.Terada M, Kelly E, Jarjour N. Increased thrombin activity after allergen challenge: a potential link to airway remodeling? American Journal of Respiratory and Critical Care Medicine. 2004;169(3):373–377. doi: 10.1164/rccm.200308-1156OC. [DOI] [PubMed] [Google Scholar]
- 28.King C, Brennan S, Thompson PJ, Stewart GA. Dust mite proteolytic allergens induce cytokine release from cultured airway epithelium. Journal of Immunology. 1998;161(7):3645–3651. [PubMed] [Google Scholar]
- 29.Asokananthan N, Graham P, Stewart D, et al. House dust mite allergens induce proinflammatory cytokines from respiratory epithelial cells: the cysteine protease allergen, Der p 1, activates protease-activated receptor (PAR)-2 and inactivates PAR-1. Journal of Immunology. 2002;169(8):4572–4578. doi: 10.4049/jimmunol.169.8.4572. [DOI] [PubMed] [Google Scholar]
- 30.Sun G, Stacey MA, Schmidt M, Mori L, Mattoli S. Interaction of mite allergens Der p3 and Der p9 with protease-activated receptor-2 expressed by lung epithelial cells. Journal of Immunology. 2001;167(2):1014–1021. doi: 10.4049/jimmunol.167.2.1014. [DOI] [PubMed] [Google Scholar]
- 31.Page K, Strunk VS, Hershenson MB. Cockroach proteases increase IL-8 expression in human bronchial epithelial cells via activation of protease-activated receptor (PAR)-2 and extracellular-signal-regulated kinase. Journal of Allergy and Clinical Immunology. 2003;112(6):1112–1118. doi: 10.1016/j.jaci.2003.08.050. [DOI] [PubMed] [Google Scholar]
- 32.Hong JH, Lee S, Kim K, et al. German cockroach extract activates protease-activated receptor 2 in human airway epithelial cells. Journal of Allergy and Clinical Immunology. 2004;113(2):315–319. doi: 10.1016/j.jaci.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 33.Miike S, Kita H. Human eosinophils are activated by cysteine proteases and release inflammatory mediators. Journal of Allergy and Clinical Immunology. 2003;111(4):704–713. doi: 10.1067/mai.2003.1332. [DOI] [PubMed] [Google Scholar]
- 34.Schmidlin F, Amadesi S, Dabbagh K, et al. Protease-activated receptor 2 mediates eosinophil infiltration and hyperreactivity in allergic inflammation of the airway. Journal of Immunology. 2002;169(9):5315–5321. doi: 10.4049/jimmunol.169.9.5315. [DOI] [PubMed] [Google Scholar]
- 35.Johnson J, Wiley R, Fattouh R, et al. Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. American Journal of Respiratory and Critical Care Medicine. 2004;169(3):378–385. doi: 10.1164/rccm.200308-1094OC. [DOI] [PubMed] [Google Scholar]
- 36.Levi-Schaffer F, Piliponsky AM. Tryptase, a novel link between allergic inflammation and fibrosis. Trends in Immunology. 2003;24(4):158–161. doi: 10.1016/s1471-4906(03)00058-9. [DOI] [PubMed] [Google Scholar]
- 37.Chokki M, Yamamura S, Eguchi H, et al. Human airway trypsin-like protease increases mucin gene expression in airway epithelial cells. American Journal of Respiratory Cell and Molecular Biology. 2004;30(4):470–478. doi: 10.1165/rcmb.2003-0199OC. [DOI] [PubMed] [Google Scholar]
- 38.Scott G, Leoparde S, Printup S, Malhi N, Seiberg M, LaPoint R. Proteinase-activated receptor-2 stimulates prostaglandin production in keratinocytes: analysis of prostaglandin receptors on human melanocytes and effects of PGE2 and PGF2alpha on melanocyte dendricity. The Journal of Investigative Dermatology. 2004;122(5):1214–1224. doi: 10.1111/j.0022-202X.2004.22516.x. [DOI] [PubMed] [Google Scholar]
- 39.D'Andrea MR, Rogahn CJ, Andrade-Gordon P. Localization of protease-activated receptors-1 and -2 in human mast cells: indications for an amplified mast cell degranulation cascade. Biotechnic & Histochemistry. 2000;75(2):85–90. doi: 10.3109/10520290009064152. [DOI] [PubMed] [Google Scholar]
- 40.Kawagoe J, Takizawa T, Matsumoto J, et al. Effect of protease-activated receptor-2 deficiency on allergic dermatitis in the mouse ear. Japanese Journal of Pharmacology. 2002;88(1):77–84. doi: 10.1254/jjp.88.77. [DOI] [PubMed] [Google Scholar]