Abstract
Background:
Calcium fortification of maize has been achieved for millenia in Central America by the process of nixtamalization. Bioavailability of calcium is, however, compromised by phytate, present in large quantities in maize kernels and only modestly reduced by nixtamalization.
Objective:
The objective of this study was to compare calcium absorption from tortilla meals prepared from low-phytate maize with that from maize with typical phytate content.
Design:
Five healthy adult women were fed two test meals of approximately 140g tortillas in lieu of breakfast at one month intervals. On one occasion the tortillas were prepared from maize with approximately 60% phytate reduction (lpa1-1) and on the other occasion from the matching isohybrid wild-type maize. 44Ca (0.3mg/kg body weight) was administered in water as an extrinsic label commencing midway through the test meal and 42Ca (0.06 mg/kg body weight) was administered intravenously immediately after the test meal. Isotope ratios of 42Ca/43Ca and 44Ca/43Ca were measured by inductively-coupled plasma mass spectrometry in urine collected as an eight-hr pool from 16-24 hrs after administration of the intravenous tracer and prepared by the oxalate precipitation method. Fractional absorption of calcium was determined by a dual isotope ratio technique.
Results:
Mean fractional absorption from tortillas prepared from the low-phytate maize was 0.50 ± 0.03 compared with a mean of 0.35 ± 0.07 from the control maize (p = 0.01).
Conclusion:
The increase in quantity of calcium absorbed could be of practical importance for calcium nutriture when intake of dairy products is limited.
Keywords: maize, phytate, low-phytate maize alleles, tortillas, calcium absorption
Introduction
Cereal grains contain only negligible quantities of calcium. However, substantial quantities of this mineral are added to maize during the process of nixtamalization (1) or liming which is the traditional method of processing maize in the preparation of tortillas in Central America. From the perspective of calcium nutriture, nixtamalization can be regarded as an early example of mineral fortification of a major food staple in Central America. Depending on the extent to which this calcium is bioavailable, maize tortillas may provide a major source of calcium for individuals whose intake of dairy products is limited.
While absorption of endogenous iron from a low-phytate maize was modestly higher than that from control maize (2), substitution of a low-phytate acid maize has not been associated with any increase in the absorption of iron added to maize flour as a fortificant (3). These observations have given additional impetus to determining if substitution of low-phytate maize effects bioavailability of calcium in maize tortillas that is derived almost entirely from the process of nixtamalization.
The hypothesis tested in this study was that fractional calcium absorption from tortilla meals prepared from maize with approximately 60% phytate reduction is significantly greater than from tortilla meals prepared from wild-type control maize.
Methods
Study Design: Fractional absorption of an extrinsic calcium stable isotope label by healthy adults was measured when administered with a test meal of tortillas prepared from low-phytate maize. In a cross-over design with wash-out period of >4 weeks, fractional calcium absorption from a test meal of tortillas prepared from a low-phytate maize was compared with that from a tortilla test meal prepared from the isohybrid wild-type control maize.
Calcium absorption was measured using an extrinsic calcium stable isotope label by a dual isotope tracer ratio technique based on measurements in the urine of the dose-adjusted ratio of enrichment with the oral extrinsic label to that for a second calcium stable isotope tracer administered intravenously (4).
Subjects: The subjects were 5 healthy free-living volunteer adult women, age range 22-29 years. The protocol was approved by the Colorado Multiple Institutional Review Board of the University of Colorado Health Sciences Center and all subjects provided their written informed consent.
Source of Maize, Preparation and Administration of Test Meals: Test meals were prepared from lpa1-1 maize with approximately 60% phytate reduction. This maize was provided by Pioneer Hi-Bred, Inc. (Dupont, Johnston, Iowa) which bred this maize under a cooperative research and development agreement with the USDA. The isohybrid wild-type maize with normal phytate content was also provided by Pioneer Hi-Bred, Inc. and was grown in the same location.
For the preparation of tortillas, ten liters of water were added to 450g maize kernels and brought to the boil. Five grams (one tsp) of powdered limestone were added and the mixture stirred. The maize was left to simmer for 4 h after which it was drained and spread out on a towel to dry for 3 h. The nixtamatalized maize was then ground in a food processor, rolled into 4 cm diameter balls, dipped in corn-oil and flattened. The tortillas were cooked on a greased skillet for 1 min. Each meal consisted of five of these tortillas which weighed approximately 35g.
Test meals were administered after an overnight fast at approximately 8am in the presence of one of the investigators. Three subjects received tortillas prepared from the low-phytate maize and the remaining two received tortillas prepared from the isohybrid wild-type maize. Following a wash-out period of 4 wks, subjects consumed the alternative test meal.
Isotope Preparation: Enriched 42Ca and 44Ca stable isotopes were obtained from Trace Science International, Inc. (Ontario, Canada) as carbonate. Enriched 44Ca was used as the orally-administered tracer and 42Ca as the intravenously administered tracer. Calcium carbonate was dissolved by adding drops of concentrated hydrochloric acid. The oral solution was prepared at a calcium concentration of 0.063 mol/L by dilution with Milli-Q water and the intravenous solution was prepared at a calcium concentration of 0.01mol/L 0.45% sterile sodium chloride. The oral solution was adjusted to pH 5.0 and the intravenous solution to ph 6.0 with sodium hydroxide. The solutions were filtered through a 0.2 μm filter to remove pyrogens. Sterile techniques were used to prepare doses for intravenous administration. Calcium concentrations were determined by atomic absorption spectrophotometry with application of correction factors for atomic weight. The oral solution was stored in plastic tubes and the intravenous solution in sealed sterile vials. The intravenous dose was tested for pyrogens immediately before use.
Isotope Administration: An accurately weighed quantity of Ca44 (approximately 0.3mg Ca/kg body weight) was administered orally in water starting approximately half way through the test meal. This method of administration has been utilized extensively by us for zinc stable isotope tracers (5). An accurately weighed quantity of Ca42 (approximately 0.06 mg Ca/kg body weight) was administered over a 10-minute time interval immediately following the test meal. Administration was performed with a 10ml syringe, 3-way stopcock and scalp vein needle into a superficial forearm vein over a 5minute interval. The syringe was flushed twice with sterile normal saline.
Sample Collection, Preparation and Analyses: Participants were instructed to completely empty the bladder immediately prior to the administration of the intravenous isotope tracer. All urine was collected for three consecutive 8-hour pooled aliquots beginning at the time of the administration of the intravenous tracer. Urine was collected directly into an acid-washed plastic bottle. Volumes were measured and a 50ml aliquot was then stored at -20 degrees centigrade.
For analysis, 5 ml urine from the third eight-hour pool (16-24 hours after isotope administration) were purified by the oxalate precipitation method (6). Urine was first centrifuged to remove particulates. Saturated ammonium oxalate was adjusted to pH 8.0 with NH4OH and 1.2 ml was added to the urine. After thorough mixing, the sample was left at room temperature overnight. It was then centrifuged at 1700 x G for 15 minutes and the supernatant was decanted. The precipitate was washed twice with Milli-Q water and dissolved in 4ml 2% HNO3. Eight milliliters of 1ppm calcium solution in 2% HNO3 was prepared from each sample. Isotope ratios of 42Ca/43Ca and 44Ca/43Ca were measured by inductively-coupled plasma mass spectrometer (ICP-MS) (Plasma Quad 3, VG Elemental, Cheshire, England). Each sample was introduced into the ICP-MS using an autosampler (ASX-500 Model 510, CETAC, Omaha, NE) and peristaltic pump (Perimax 12, CPETEC, Erding, Germany). In order to minimize argon derived isobaric and polyatomic interference, the plasma was operated at low power. Instrumental parameters for cool plasma operation are given in Table 1.
Table 1.
Inductively-Coupled Plasma Mass Spectrometer and Ca Isotope Data Acquisition Parameters
| Argon Gas Flow: |
| Outer gas flow: 12.0 L/min |
| Intermediate gas flow: 0.50 L/min |
| Nebulizer gas flow: 1.00 L/min |
| Forward Power: 700 W |
| Temperature of Pneumatic Nebulizer: 4 °C |
| Sample Flow Rate: 1 ml/min |
| Data Acquisition: |
| Dwell Time: 42Ca: 4ms, 43Ca: 5ms, 44Ca: 3ms |
| Point per Peak: 1 |
| Sweeps: 2280 |
| Dead Time: 50ns |
| Number of Replicates: 10 |
Two percent HNO3 and a 1ppm natural abundance calcium standard were used to optimize ICP-MS tuning to attain the lowest ion count for 2% HNO3 blank and the highest count rate for the calcium standard. With a 44Ca count rate of 300,000-400,000 per second (CPS) from the 1ppm natural calcium standard, the ICP-MS can be tuned to produce a 2% HNO3 signal of <0.4% of the 1ppm Ca signal for isotopes 42 Ca, 43Ca and 44Ca. A natural calcium standard was analyzed after every 6 urine samples and 2% HNO3 was analyzed after every 12 samples. The results were used to reduce any effect of instrumental drift on measured ratios. The 2% HNO3 count rate was subtracted from urine sample count rates. The relative standard deviation (RSD) for the analysis of 10 replicates was <0.3% for the ratios 42Ca/43Ca and 44Ca/43Ca. The calcium tracer enrichments were calculated from the measured isotope ratios using an algorithm that takes into account the contribution to each isotope signal from both isotopically-enriched tracers and the natural calcium in the sample. For each isotope tracer used, enrichment is defined as all the calcium in the sample from that particular tracer divided by the total amount of calcium in the sample.
Weights of test meals consumed were recorded. Tortillas from each test meal were collected and homogenized. Weighed aliquots were digested and the calcium content was determined by atomic absorption spectrophotometry (7). HPLC was used to directly measure phytate (8).
Data Processing and Statistical Analyses: Data were analyzed using GraphPad Prism, version 4.00 for Windows (GraphPad Software, San Diego, CA, www.graphpad.com). Calcium intake (mean ± SD) was calculated per g tortilla and per test meal. Calcium: phytate molar ratios of the test and control meals were calculated.
Means for fractional calcium absorption were determined for the low-phytate and for the wild-type maize tortilla meals. Calcium absorption from the low-phytate maize tortilla meals was compared with that from the wild-type control maize tortilla meals using two-tailed, paired comparison t-test.
Results
Results are presented as means ± SD. The calcium contents of the low-phytate and control tortillas were not significantly different (p = 0.90) and results were combined to give a mean (± SD) calcium concentration of 1.00 ± 0.39 mg Ca/g wet weight tortilla). The quantity of tortilla consumed averaged 140 g/test meal, with a mean calcium intake of 140 mg. Phytate concentrations of the low-phytate and control tortillas were 1.56 and 3.0 mg/g, respectively, giving corresponding Ca:phytate molar ratios of 10.9 and 4.9.
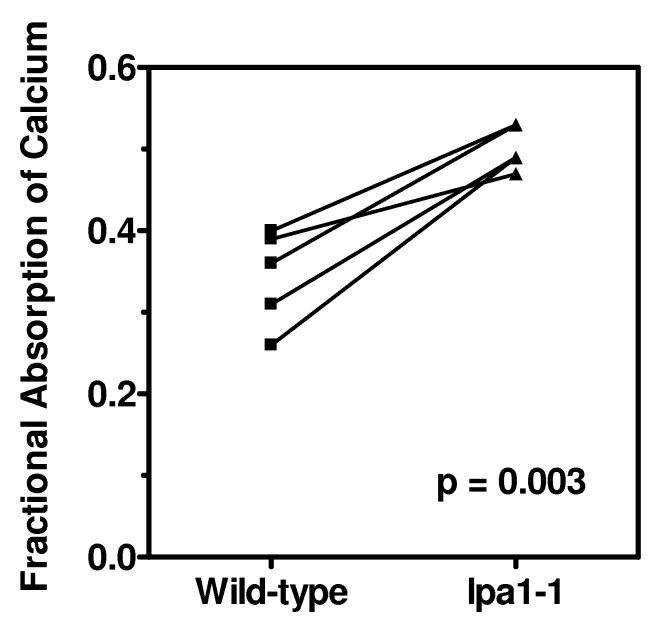
All individual subjects had higher fractional absorption of calcium from the lpa1-1 maize tortilla meals than from the control maize tortilla meals (Figure 1). Mean fractional absorption of calcium from the low-phytate maize tortilla meals was 0.50 ± 0.03 compared with a mean of 0.35 ± 0.07 from the tortilla meals prepared from the isohybrid wild-type control maize (p < 0.01).
Figure 1.
Comparison of fractional calcium absorption from tortillas prepared with a 60% phytate reduced maize (lpa1-1) or isohybrid wild-type maize in individual subjects.
Discussion
Phytate chelates calcium strongly. Calcium-phytate complexes are quite soluble at acidic pH, but have only very limited solubility at the neutral or alkaline pH of the small intestine (9). Phytate was reported to have an inhibitory effect on calcium absorption in rats as early as 1934 (10). Subsequent reports of the effects of phytate on calcium absorption in rodents have been conflicting (9-17), which, it has been suggested, may be attributable to the presence of intestinal phytases in the rodent (18). Substitution of low-phytate ceral grains in feeds for chicks, pigs and fish has been associated with improved calcium nutrition in each species (19)(20)(21). In the human, destruction of phytate in wheat flour was shown to improve calcium retention in 1942 (22). The inhibitory effect of phytate on calcium absorption in the human has subsequently been confirmed (23) (24) and calcium absorption from low-phytate soybeans has been reported to be significantly higher than that from high-phytate soybeans (18). Even in the human, however, the adverse effect of phytate on calcium absorption has excited no (25) or relatively little (26) attention in reviews.
In the current study, the difference in calcium absorption from tortilla meals prepared with the low-phytate maize was in the same direction but of greater magnitude than that for a low-phytate vs. high-phytate whole cooked soybeans (18). The calcium content of the tortillas prepared for this study was comparable to that found by other investigators (1). With a typical tortilla wet weight of approximately 40g, the quantity ingested with each tortilla was about 40 mg Ca. In our own experience in the Western Highlands of Guatemala, adult women typically consume at last 15-20 tortillas (∼ weight 40 g each) per day providing at least 500mg calcium per day. The increased absorption found in the low-phytate maize tortillas would contribute an additional 6 mg absorbed Ca per tortilla or 90-120 mg Ca/day for typical maize intakes. Thus, with a limited intake of dairy products, tortillas provide the major source of dietary calcium. This alone, however, is not typically sufficient to match the Adequate Intake (AI) of the Food and Nutrition Board, Institute of Medicine (27) which indicates that any strategy that increases the bioavailabilty of this calcium will make a useful contribution to calcium status. Even in North America, tortillas prepared from nixtamalization of maize can provide a useful alternative source of calcium for subjects who do not consume dairy products and, again, the superior absorption from a low-phytate maize could be advantageous.
We have shown previously that fractional absorption of zinc from tortillas prepared from maize with two different levels of phytate reduction (but probably a mutation of the same allele) is higher than that from tortillas prepared from the corresponding wild-type maize with typical phytate content (28, 29). Others have demonstrated a modest increase in iron absorption (2). Especially when taken together, these data encourage evaluation of the efficacy and effectiveness of a change in agricultural practice, i.e. the use of lower phytate maize, to improve mineral nutriture in low income populations that depend on maize as their principal food staple.
Acknowledgements
KM Hambidge, NF Krebs, JL Westcott, S Lei, LV Miller and V Raboy participated in study design and data interpretation. S Lei and KL Peterson were responsible for laboratory analyses. Data were analyzed by JL Westcott. The manuscript was drafted by KM Hambidge. None of the authors have any conflict of interest with this study.
Footnotes
Supported by the USDA/ARS, NRICGP # 99000663. Also supported in part by The Thrasher Research Fund and CNRU #5P30 DK48520.
References
- 1.Bressani R, Turcios JC, de Ruiz ASC. Nixtamalization effects on the contents of phytic acid, calcium, iron and zinc in the whole grain, endosperm and germ of 11 maize varieties. Food Sci and Tech Interal. 2002;8:81–6. [Google Scholar]
- 2.Mendoza C, Viteri F, Lonnerdal B, Young KA, Raboy V, Brown KH. Effects of genetically modified, low-phytic acid maize on absorption of iron from tortillas. Am J Clin Nutr. 1998;68:1123–7. doi: 10.1093/ajcn/68.5.1123. [DOI] [PubMed] [Google Scholar]
- 3.Mendoza C, Viteri FE, Lonnerdal B, Raboy V, Young KA, Brown KH. Absorption of iron from unmodified maize and genetically altered, low-phytate maize fortified with ferrous sulfate or sodium iron EDTA. Am J Clin Nutr. 2001;73:80–5. doi: 10.1093/ajcn/73.1.80. [DOI] [PubMed] [Google Scholar]
- 4.Yergey AL, Abrams SA, Vieira NE, Aldroubi A, Marini J, Sidbury JB. Determination of fractional absorption of dietary calcium in humans. J Nutr. 1994;124:674–82. doi: 10.1093/jn/124.5.674. [DOI] [PubMed] [Google Scholar]
- 5.Krebs N, Miller LV, Naake VL, et al. The use of stable isotope techniques to assess zinc metabolism. J Nutr Biochem. 1995;6:292–307. [Google Scholar]
- 6.Patterson KY, Veillon C, Hill AD, Moser-Veillon PB, O’Haver TC. Measurement of calcium stable isotope tracers using cool plasma ICP-MS. J Analytical Atomic Spectrometry. 1999;14:1673–1677. [Google Scholar]
- 7.Trudeau DL, Freier EF. Determination of calcium in urine and serum by atomic absorption spectrophotometry (AAS) Clin Chem. 1967;13:101–114. [PubMed] [Google Scholar]
- 8.Rounds MA, Nielsen SS. Anion-exchange high-performance liquid chromatography with post-column detection for the analysis of phytic acid and other inositol phosphates. J Chromatograhpy A. 1993;653:148–152. doi: 10.1016/0021-9673(93)80404-V. [DOI] [PubMed] [Google Scholar]
- 9.Zhou JR, Erdman JW., Jr. Phytic acid in health and disease. Crit Rev Food Sci Nutr. 1995;35:495–508. doi: 10.1080/10408399509527712. [DOI] [PubMed] [Google Scholar]
- 10.Harrison D, Mellenby E. Phytate acid and the rickets-producing action of cereal. Biochem. 1934;28:517. doi: 10.1042/bj0331660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lonnerdal BSA-S, Sandstrom B, et al. Inhibitory effects of phytic acid and other inositol phosphates on zinc and calcium absorption in suckling rats. J Nutr. 1989;119:211–14. doi: 10.1093/jn/119.2.211. [DOI] [PubMed] [Google Scholar]
- 12.Miyaznwa E, Yoshida T. Effects of dietary levels of phytate and inorganic phosphate on phytate breakdown and absorption of calcium and magnesium in rats. Nutr Res. 1991;11:797. [Google Scholar]
- 13.Graf E, Eaton JW. Effects of phytate on mineral bioavailability in mice. J Nutr. 1984;114:1192–8. doi: 10.1093/jn/114.7.1192. [DOI] [PubMed] [Google Scholar]
- 14.Churella HR, Vivian VM. Effect of phytic acid level in soy-protein based infant formulas on mineral availability in the rat. J Agric Food Chem. 1989;37:1352. [Google Scholar]
- 15.Ranhotra GS, Gelroth JA, Torrence FA, Bock MA, Winterringer GL. Bread (white and whole wheat) and nonfat dry milk as sources of bioavailable calcium for rats. J Nutr. 1981;111:2081–6. doi: 10.1093/jn/111.12.2081. [DOI] [PubMed] [Google Scholar]
- 16.Mason AC, Weaver CM, Kimmel S, Brown RK. Effect of soybean phytate content on calcium bioavailability in mature and immature rats. J Agric Food Chem. 1993;41:246. [Google Scholar]
- 17.Poneros AG, Erdman MW. Bioavailability of calcium from sesame seeds, almond powder, whole wheat bread, spinach and non-fat dry milk in rats. J Food Sci. 1989;54:150. [Google Scholar]
- 18.Heaney RP, Weaver CM, Fitzsimmons ML. Soybean phytate content: effect on calcium absorption. Am J Clin Nutr. 1991;53:745–7. doi: 10.1093/ajcn/53.3.745. [DOI] [PubMed] [Google Scholar]
- 19.Veum TL, Ledoux DR, Bollinger DW, Raboy V, Cook A. Low-phytic acid barley improves calcium and phosphorus utilization and growth performance in growing pigs. J Anim Sci. 2002;80:2663–70. [PubMed] [Google Scholar]
- 20.Overturf K, Raboy V, Cheng ZJ, Hardy RW. Mineral availability from barley low phytic acid granins in rainbow trout (oncorhynchus mykiss) diets. Aquaculture Nutrition. 2003;9:239–246. [Google Scholar]
- 21.Ertl DS, Young KA, Raboy V. Plant genetic approaches to phosphorus management in agricultural production. Journal of Environ Qual. 1998;27:299–304. [Google Scholar]
- 22.McCance R, Widdowson E. Mineral metabolism of dephytinized bread. J Physiol. 1942;101:304. doi: 10.1113/jphysiol.1942.sp003984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knox TA, Kassarjian Z, Dawson-Hughes B, et al. Calcium absorption in elderly subjects on high- and low-fiber diets: effect of gastric acidity. Am J Clin Nutr. 1991;53:1480–6. doi: 10.1093/ajcn/53.6.1480. [DOI] [PubMed] [Google Scholar]
- 24.Reinhold JG, Nasr K, Lahimgarzadeh A, Hedayati H. Effects of purified phytate and phytate-rich bread upon metabolism of zinc, calcium, phosphorus, and nitrogen in man. Lancet. 1973;1:283–8. doi: 10.1016/s0140-6736(73)91538-9. [DOI] [PubMed] [Google Scholar]
- 25.Bronner F. Calcium absorption--a paradigm for mineral absorption. J Nutr. 1998;128:917–20. doi: 10.1093/jn/128.5.917. [DOI] [PubMed] [Google Scholar]
- 26.Allen LH. Calcium bioavailability and absorption: a review. Am J Clin Nutr. 1982;35:783–808. doi: 10.1093/ajcn/35.4.783. [DOI] [PubMed] [Google Scholar]
- 27.Food and Nutrition Board, National Research Council . Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Flouride. National Academy Press; Washington, DC: 1997. [PubMed] [Google Scholar]
- 28.Adams CL, Hambidge M, Raboy V, et al. Zinc absorption from a low-phytic acid maize. Am J Clin Nutr. 2002;76:556–9. doi: 10.1093/ajcn/76.3.556. [DOI] [PubMed] [Google Scholar]
- 29.Hambidge KM, Huffer JW, Raboy V, et al. Zinc absorption from low-phytate hybrids of maize and their wild-type isohybrids. Am J Clin Nutr. 2004;79:1053–9. doi: 10.1093/ajcn/79.6.1053. [DOI] [PubMed] [Google Scholar]