Abstract
The Plasmodium falciparum sporozoite infects different types of cells in a mosquito's salivary glands and human epithelial and Kuppfer cells and hepatocytes. These become differentiated later on, transforming themselves into the invasive red blood cell form, the merozoite. The ability of sporozoites to interact with different types of cells requires a wide variety of mechanisms allowing them to survive in both hosts: mobility, receptor-ligand interactions with different cellular receptors, and transformation and development into other invasive parasite forms, which are vitally important for parasite survival. Sporozoite complexity is reflected in the large quantity of proteins that can be expressed. Some of them have been extensively studied, such as CSP, TRAP, STARP, LSA-1, LSA-3, SALSA, SPECT1, SPECT2, MAEBL, and SPATR, due to their importance in infection and their potential use as vaccines. Our work has been focused on the search for the molecular mechanisms of parasite-host cellular receptor-ligand interactions by identifying amino acid sequences and the critical binding residues from these proteins relevant to parasite invasion. Once such sequences have been identified, it will be possible to modify them to induce a strong immune response against P. falciparum in the experimental Aotus monkey model. This all leads towards developing multistage, multicomponent, subunit-based vaccines that will be effective in eradicating or controlling malaria caused by P. falciparum.
INTRODUCTION
Infection caused by Plasmodium falciparum (the most lethal form of human malaria) has been difficult to control and/or eradicate due to the parasite's tremendous complexity. The P. falciparum sporozoite is able to invade both mosquito and vertebrate host cells. This ability to invade different target cells could be due to sporozoites using different invasion mechanisms involving the participation of a still-unknown number of parasite proteins. It has thus become necessary to identify and study those proteins involved in these invasion processes that could be used in developing a vaccine leading to controlling and/or eradicating this disease. A viable and novel alternative lies in developing multistage, multicomponent, synthetic vaccines. However, this requires a broad and deep knowledge of the parasite's molecular biology. Vaccines based on the antigens identified to date have generally presented discouraging results. The SPf66 synthetic vaccine has been the only vaccine to present significant protection (∼35%) in different populations. Our experience with the SPf66 synthetic vaccine has indicated that it is possible to obtain effective vaccines against P. falciparum malaria based on peptide sequences derived from relevant parasite proteins involved in infection. Knowledge of the P. falciparum genome represents a valuable tool for this purpose, but, as expected, the number of proteins that may be expressed during the sporozoite stage easily exceeds the number of proteins that can be expressed during parasite stages able to infect red blood cells or during sexual stages combining in a mosquito's middle intestine. It must be remembered that sporozoites are able to infect a mosquito's salivary gland cells, migrate through mammalian cells, and invade human liver cells. These processes imply the participation of a still-undetermined number of parasite proteins. Some parasite proteins that are considered to be relevant in hepatic cell infection have been identified and studied to date, but such proteins represent a small percentage of the total number of proteins that could express the sporozoite. The above-mentioned findings suggest that there is still much to be learned about the parasite and that as more knowledge is gained, we become closer to developing immunoprophylactic and therapeutical methods for controlling malaria caused by P. falciparum and being able to eradicate it.
THE MALARIAL PARASITE
Malaria, mainly caused by P. falciparum, is considered to be one of the diseases with the greatest impact on public health worldwide, with around 500 million people suffering the disease and 2 to 3 million people dying each year, especially children less than 5 years of age in sub-Saharan Africa (116).
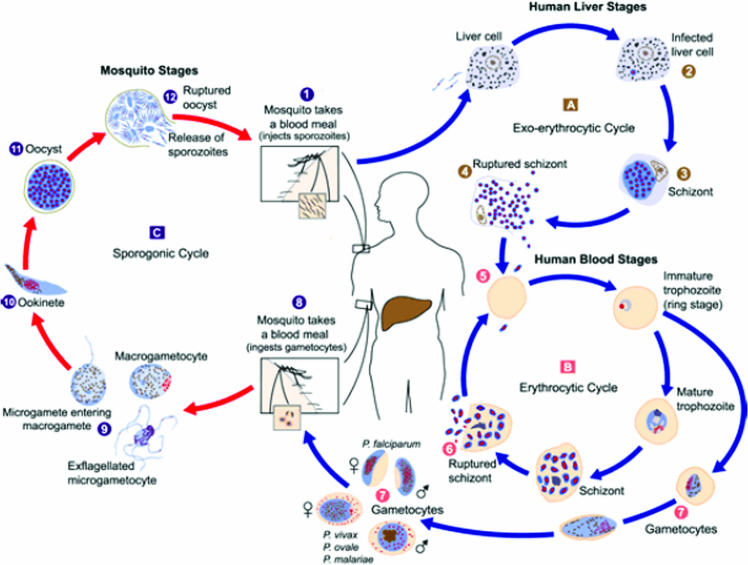
The life cycle of the P. falciparum parasite consists of an exogenous sexual phase, where male and female gametes combine within the mosquito's middle gut; this is followed by an exogenous asexual phase (sporogony), where the parasite multiplies within a mosquito's gut, which is then followed by an endogenous asexual phase (schizogony) leading to parasite multiplication in the vertebrate host (Fig. 1). The latter phase includes the parasite's development in parenchymal liver cells (preerythrocytic schizogony) and red blood cells (RBC) (erythrocytic schizogony) (115a).
FIG. 1.
Plasmodium falciparum life cycle in the human host and the Anopheles mosquito. The parasite's stages in the human host and the invertebrate host can be observed. The parasite forms interact with different classes of both human and mosquito cells. (Taken from the CDC website for laboratory identification of parasites [http://www.uni-tuebingen.de/modeling/Mod_Malaria_Cycle_en.html].)
The parasite's life cycle in the human host begins by sporozoites being inoculated into a host's skin through the bite of an infected mosquito; these sporozoites (having a larva-like morphology) are rapidly transported to the liver once they reach the bloodstream, and they are retained there by specific molecules, ensuring that the parasite does not become eliminated in the spleen (Fig. 1). The migration of these sporozoites through hepatic cells to infect hepatocytes leads to intrahepatic development that (by proliferation and differentiation) culminates in the liberation of merozoites, which have now adopted a lemon-like morphology, capable of infecting erythrocytes (44, 63).
The parasite's erythrocytic cycle begins with merozoite recognition of, binding to, and invasion of RBC. Once erythrocytes have been invaded, the parasite is found inside the parasitophorous vacuole. The parasite's intraerythrocytic development goes through different stages (ring, trophozoite, and schizont); new merozoites are finally developed, which are liberated following erythrocyte lysis so as to infect new erythrocytes (6). Some merozoites develop sexual parasite morphologies (male and female gametes), which are transported to a mosquito's stomach while ingesting a human blood meal. Once in the stomach, the parasite undergoes a series of transformations, culminating in the development of new sporozoites that invade the salivary glands so that they can then be inoculated into another host (65).
Many studies aimed at finding control mechanisms have been carried due to the capital importance of understanding the agent's molecular characteristics (Plasmodium parasites). This has led to the recent description of the P. falciparum parasite genome (49). However, the study of interactions between receptor molecules on the cells that they infect and receptor-ligand interactions of the sporozoite molecules with their host cells at the molecular level remains open. This study is aimed at summarizing known interactions at the functional and molecular level due to their great implication in a logical approach for designing and developing vaccines for controlling this deadly disease.
The structural characteristics of the sporozoite will thus be analyzed, as will biological modifications made to the mosquito vector and its activities, the processes occurring after a mosquito bites a vertebrate host (including an analysis of the mobility mechanisms used for hepatocyte invasion), a thorough molecular analysis of the different proteins and peptides involved in these processes, and the immunological activities induced by some of these peptides in Aotus nancymaae monkeys as a methodology for showing the feasibility of logically developing a multiepitopic sporozoite synthetic antimalarial vaccine based on these subunits.
A Sporozoite's Biological Characteristics
The malarial parasite (Plasmodium spp.) belongs to the phylum Apicomplexa, which includes intracellular pathogens such as Toxoplasma, Cryptosporidium, Eimeria, Babesia, and Theileria species. All Apicomplexa are intracellular parasites, with most growing and replicating within a nonphagosomal parasitophorous vacuole, a compartment bound to the membrane and segregated from most intracellular traffic routes (81).
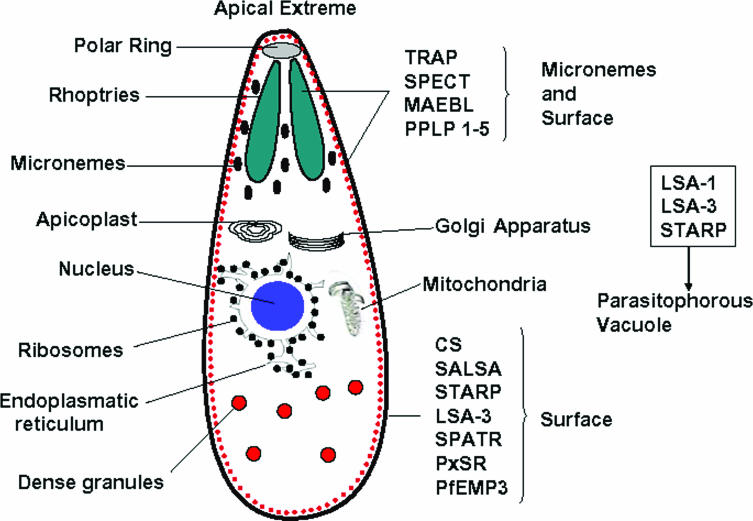
Some Apicomplexa have a group of organelles (called rhoptries, micronemes, the apical polar ring, and conoids) that form their apical complex (Fig. 2). The rhoptries and micronemes are the only secretor organelles that contain products required for mobility, adhesion, host cell invasion, and parasitophorous vacuole formation (88). The Apicomplexa have another unique structural characteristic, called the apicoplast (similar to a chloroplast), which is an essential organelle for parasite survival (81). These parasites are surrounded by a pellicle, which is a structure formed by the plasma membrane and an internal membrane complex (IMC) found within the cell, intimately associated with several cytoskeleton elements such as actin, myosin, microtubules, and a network of filament-like proteins involved in sporozoite mobilization (81) (Fig. 2).
FIG. 2.
Diagram of a sporozoite's internal structure and some of the main molecules associated with it. The localizations of the different sporozoite proteins that have been related to parasite survival can be observed.
Apicomplexan proliferation occurs due to host cell invasion followed by parasite growth and cell division until the host cell is lysed. Parasites become liberated and do not show growth or extracellular division, meaning that they must speedily invade other host cells (6). Apicomplexa are haploid for most of the cycle and evolve to forms that invade different tissues and cells by proliferation and differentiation. Differentiation could produce gametes that undergo male-female fusion to produce a diploid zygote, transforming into another haploid organism by meiosis. This differentiation allows the infection of organisms that serve as vectors for these parasites to be transmitted from one host to another (6, 81).
Sporozoites in the Mosquito Vector
Following ingestion of the infected blood meal by a female Anopheles mosquito, male (♂) and female (♀) immature gametes circulating in an infected vertebrate host's bloodstream reach a mosquito's stomach, where microgametes become differentiated into macrogametes due to the presence of factors such as temperature, environmental change, metabolite differences, etc. The male gamete (♂) undergoes three DNA replication cycles, and eight flagella are assembled in the cytoplasm, where each flagellum becomes bound to one of the genome's eight haploid copies. Meanwhile, female gametes (♀) become stripped when they get rid of the RBC envelops. The macrogamete (♀) nucleus moves towards the surface, where it forms a small protuberance. Exflagellation consists of microgametes (♂) becoming formed, expelled, and liberated; they then penetrate the macrogametes (♀), forming zygotes (♂♀) (http://www.brown.edu/Courses/Bio_160/Projects1999/malaria/ldpg.html) (Fig. 1).
Once zygotes are formed, they in turn become ookinetes, an invasive mobile diploid form that undergoes meiosis, finishing with the formation of four haploid genomes within a nucleus. The apical complex is found within the upper part of the ookinete, where the secretor organelles are found (i.e., the rhoptries and micronemes). This ookinete migrates through the liquid of the alimentary bolus and passes through the defensive layer and the microvillar network of the peritrophic matrix so that it can then invade epithelial cells in a mosquito's stomach (70, 115a) (Fig. 1).
Once an ookinete has passed through these cells, it binds to the basal lamina and loses its mobility, and a trophic form (called an oocyst), which is able to replicate, becomes differentiated. The hemolymph provides the nutrients for an oocyst to develop; an oocyst's nucleus is elongated and polyploid, containing two parental and two recombinant genotypes. The genome rapidly divides, forming 6,000 to 8,000 haploid nuclei, after which the cytoplasm subdivides into multiple grooves originated by endoplasmatic reticulum expansion, and when the oocyst has matured, it contains hundreds of sporozoites packed into lobes. When this oocyst explodes, liberating thousands of sporozoites, it is not known whether this is due to passive rupture of the lobe or the sporozoite causing its active degradation (http://www.brown.edu/Courses/Bio_160/Projects1999/malaria/ldpg.html) (7, 8, 9, 117) (Fig. 1).
Once the sporozoites have passed through the basal lamina covering the salivary glands, they come into contact with the plasma membranes of the secretor cells (94). The mechanism for penetrating the basal lamina remains unknown; however, the circumsporozoite protein (CS) has been detected in the basal lamina following parasite penetration (94). Studies using a library of peptides fused to phages have shown that the salivary gland midgut (SM1) peptide (which has the sequence PCQRAIFQSNCN) specifically binds to the luminal side of a mosquito's stomach and the distal lobes of salivary glands (51).
Sporozoites must subsequently pass through its secretor cells to reach the secretory cavity (45, 94). After passing through this cavity, they present some of the following characteristics: (i) they can infect, (ii) they can induce an immune response in a mammalian host, and (iii) they present a type of mobility where speed and movement patterns may be modulated to be different from that found in vitro (45).
There is evidence that the three most studied sporozoite surface proteins are involved in the invasion of a mosquito's salivary glands, i.e., circumsporozoite (CS), thrombospondin-related anonymous protein (TRAP), and membrane apical erythrocyte binding-like (MAEBL) surface protein. This is based on the fact that CS recombinant proteins specifically bind to the median and distal lobes of such salivary glands and that a peptide in CS (86LRKPKHKKLKQPADG100) region I blocks CS recombinant protein and sporozoite binding to salivary glands (33, 71, 86, 119). It has also been reported that TRAP-mutated Plasmodium berghei sporozoites (by homologous recombination) present normal morphological characteristics but weakly invade the salivary glands (120). Studies with P. berghei sporozoites that do not express the MAEBL protein have also indicated that this protein is essential for sporozoite salivary gland infection (71). Since molecular interactions are essential for sporozoite invasion of vertebrate host cells (and therefore for vaccine development), these protein interactions must be analyzed at the molecular level.
The Sporozoite in the Vertebrate Host
Infection of a vertebrate host begins with a female Anopheles mosquito bite, where 20 to 200 sporozoites are inoculated into the skin by the bite; they stay there for a short time, possibly permeating the epidermis to enter the bloodstream (68). Several studies have suggested that the number of sporozoites inoculated does not depend on the sporozoite load within a mosquito's salivary glands but rather is correlated with active colonization of the salivary channels (45). It has been reported that sporozoites inoculated by biting mosquitoes are more infective than those extracted from the salivary glands and injected intravenously (70).
The mosquitoes' salivary glands liberate different soluble components during the first phase, such as antihistamines, vasodilators (tachykinin), anticoagulants (thrombin), platelet aggregation inhibitors, and immunomodulators, to facilitate blood ingestion, thus contributing to sporozoite survival and thereby facilitating their inoculation (5, 70, 112).
Mobilization To Reach the Liver
Once the sporozoites have reached the blood, they are transported via the hepatic artery or the portal vein towards the hepatic sinusoids, which are composed of highly fenestrated endothelial cells and specialized macrophages, the Kupffer cells. Sporozoite retention in the hepatic sinusoid could possibly be mediated by the main or most abundant sporozoite surface proteins (CS and TRAP). It has been reported that recombinant CS proteins bind to heparan sulfate proteoglycans (HSPGs) from the basolateral cell surface of hepatocytes in the Disse space and that this interaction occurs between the CS protein's conserved I and II-plus regions and heparin-like oligosaccharides and/or heparan sulfate (4, 23, 24, 95, 106).
The nature of the HPSG receptor remains unknown; these receptors are widely distributed throughout the extracellular matrix and on the cell surface. Hepatic HPGSs include two members of the Sydecan family (68), which are integral type I membrane proteins; these could function as coreceptors. Sydecan 2 is the molecule that most resembles the CS protein receptor; it contains a large percentage of heparin with highly sulfated structures at the extreme distal glycosaminoglycan chains, where heparin is the most efficient inhibitor of CS binding to HepG2 cells (70). In favor of these findings, is has been found that CS binding to liver sections and HepG2 cells becomes inhibited when these cells are treated with heparitinase, an enzyme that removes sugar from the backbone (119). Inhibition studies with glycosaminoglycans have shown that decasaccharides isolated from heparin block CS interactions with HepG2 cells (70).
Following the initial retention in the hepatic sinusoid, sporozoites migrate through the sinusoidal cellular layer to be able to infect the hepatocytes. Ishino et al. proposed that the sinusoidal barrier is not perfect, suggesting that sporozoites use extracellular routes for reaching the hepatocytes, migrating between sinusoidal endothelial cells or via fenestrations characteristic of sinusoidal epithelial cells (63, 64, 135). This hypothesis is supported by the fact that both wild-strain sporozoites and those possessing the sporozoite micronemal protein essential for cell transversal (SPECT2)-interrupted gene can migrate through a number of separations created in rat Kupffer cell-depleted endothelial cells (63). However, the parasite's size exceeds that of the fenestrations presented in the liver (95).
Kupffer cell phagocytosis inhibition assays (using silica particles) have shown that an increase in the time that sporozoites stay in the blood produces a reduced number of exoerythrocyte forms. However, this result is controversial due to the finding that silica induces an increase in interleukin-6, which is known to be an inhibitor of sporozoite invasion and schizont development (95). Inhibition assays performed using gadolinium chloride showed that such treatment does not significantly affect P. yoelii or P. berghei sporozoite phagocytosis in Kupffer cells (95).
Electron microscopy studies have shown that P. yoelii sporozoites are surrounded by a vacuole within Kupffer cells, suggesting that sporozoites pass through these cells to reach the hepatocytes (95, 135). P. berghei sporozoites have also been observed to be surrounded by a vacuolar membrane within the macrophages without presenting structural degeneration (31, 44, 110), suggesting that sporozoites do not become digested by Kupffer cells. However, it has been suggested that the role of Kupffer cells could be different in animals immunized with sporozoites, where there could be destruction and removal of antibody-coated sporozoites (31).
Sporozoite migration through host cells could affect the host's immune response and interfere with it; once a sporozoite has passed through the Kupffer cells, it has to cross the Disse space to pierce the hepatocyte's basolateral membrane and then go through it (44, 95). Other authors proposed that Kupffer cells use their scavenger function for removing sporozoites from blood by phagocytosis; however, a small number of parasites rapidly traverse the Kupffer cells to escape destruction by processes such as lysosome digestion and thus invade the hepatocytes (95).
Another hypothesis suggests that sporozoites could bind to a receptor and thus directly invade hepatocytes (110, 119). This is supported by the fact that CS protein (the most abundant sporozoite surface protein) binds to hepatocyte microvilli. These microvilli are the part of a hepatocyte exposed to the circulation and are separated from the endothelial cell layer by the Disse space (24, 119). It has also been suggested that hepatocyte Syndecan 2 protein HSPG chains become extended by means of the sinusoidal fenestrated endothelium and bind to thrombospondin-related (TSR) domain positively charged residues in CS protein (43, 68). Parasites bound to HSPG could be spilled across the sinusoidal layer, probably through Kupffer cells, and enter the Disse space, where they react with the hepatocyte basolateral membrane (24, 68).
Sporozoite Mobility
Three types of sporozoite movement allow the sporozoite to migrate through the liver to reach the hepatocytes, which they then infect (135).
Sporozoite mobility due to gliding.
Sporozoite mobility due to gliding consists of the sporozoite's circular movement on a glass surface in the presence of sera. This movement could be represented as extracellular locomotion-like migration on the cell surface and through the cellular matrix. It could also be involved in migration through subcutaneous tissue towards the blood vessels following a mosquito bite (135).
Sporozoite mobility is based on machinery characteristic of apicomplexan parasites; this does not imply changes in the form of the cell and is not based on flagella or cilia (81, 135). Micronemes participate in this movement via adhesion proteins (adhesins) secreted by the apical pole, which then become redistributed all over the surface (135). A translocation is presented when adhesins interact with an external substrate that produces traction and thus movement; this is why mobility is considered to be substrate dependent (53, 111, 135). This form of mobility seems to be essential for sporozoite infection of liver cells (82, 83). Inhibiting gliding mobility with an actin-depolymerizing substance (cytocalasin) effectively blocks the invasion of these cells; however, it is not known whether the effect of cytocalasin is due to the inhibition of parasite mobility or other effects on the parasite itself or the host cell (82).
P. falciparum sporozoite gliding is associated with a redistribution of the parasite's surface proteins towards the posterior region, as has been demonstrated for the CS protein, which is secreted at the parasite's apical extreme, translocated all over its surface by an actin-dependent process, and deposited in the posterior part. Sporozoite gliding within this context is produced by the binding of surface proteins to the substrate, resulting in a characteristic spiral movement that can be seen by immunostaining of the CS protein (82, 114). Such mobility is inhibited by sporozoite incubation with monoclonal antibodies against CS protein; however, this inhibition does not eliminate this protein secretion. When this secretion is inhibited by treatment with calcium chelators or secretion inhibitors, it also inhibits parasite mobility (82).
TRAP is the other protein secreted during gliding (21). Plasmodium lines containing TRAP protein mutations do not present this mobility and are incapable of invading hepatocytes, suggesting that gliding mobility is also a relevant factor in sporozoite infection of hepatocytes (69, 120). Genetic studies have indicated that invasion and mobility require the participation of different extracellular components where certain alterations in adhesion domains deteriorate sporozoite invasion ability but do not affect gliding (82). Recent studies have suggested that TRAP could be the bridge between the parasite's cytoskeleton and receptors on the target cell or in the extracellular matrix (21, 82).
Invasive Apicomplexa are generally surrounded by microtubules supporting a triple membrane layer consisting of an external, a central, and an internal membrane forming the IMC (81). Sporozoite gliding occurs via the actin-myosin motor, localized in the space between the plasma membrane and the IMC (21). Myosin is bound to the IMC by a protein called myosin A tail domain-interacting protein (MTIP), which is in turn bound to the myosin docking protein (13). The TRAP intracytoplasmatic domain is bound to the F-actin complex by means of glycolytic enzyme aldolase. The intracellular motor moves TRAP backwards and, due to the protein's extracellular portion, produces a forward parasite movement when becoming fixed to the substrate (21, 119).
Mobility for traversing cells.
The second type of sporozoite movement is used by the parasite for migration through cells by breaking cellular barriers, leading to membrane rupture (135). This is known as active transcytosis, which is the ability to migrate through a cell. Mota et al. (83) used an elegant assay to show that sporozoites can traverse HepG2 cells by rupturing the cellular membrane, followed by its later repair. The ability of sporozoites to migrate through different types of vertebrate cells provides them with the ability to gain access to the cells that they are about to invade and then use them during their intracellular development.
Mobility for invasion.
The third type of movement can be defined as mobility for invasion; this movement involves the formation of the parasitophorous vacuole and parasite gliding within it (135). In contrast with mobility for traversing cells, this type of movement does not imply a rupturing of the host cell membrane, as this is contiguous with the parasitophorous vacuole's membrane. This allows the host cell to remain intact, allowing the parasite to develop into an erythrocyte invasive form within it (135).
Hepatocyte Invasion
After crossing the Disse space, sporozoites migrate through several hepatocytes before invading and developing to produce hepatic schizonts and merozoites. It seems that invasion processes imply specific recognition between host cell receptors and sporozoites; however, it has been suggested that additional host cell factors are required for parasite invasion (82).
Studies carried out by Mota et al. (83) have indicated that sporozoites could pass through Hep1-6 cells in a process that is rapidly followed by membrane repair (named sporozoite transcytosis) and that passage through cells is a prerequisite for enabling them to invade the appropriate hepatic cell (83, 84). Electron microscopy studies have demonstrated both free sporozoites in the cytosol and sporozoites surrounded by a parasitophorous vacuole, suggesting that sporozoites could traverse hepatic cells in a free form or surrounded by a parasitophorous vacuole (83). Even though there is controversy concerning whether sporozoites are found in a free form in the host cell cytosol, the indisputable fact is that there is evidence that sporozoites migrate through the cells before reaching the hepatic cell (invasion) target, which allows parasite intrahepatic development (82).
It is thought that this migration makes sporozoites infection competent by activating the secretion of products involved in the intracellular formation of the parasitophorous vacuole (83). It has been proposed that sporozoites become stimulated by proteins present in the cytoplasm when traversing them. Sporozoite migration through hepatocytes triggers the secretion of hepatocyte growth factor, activating the hepatocyte growth factor receptor and thus producing increased liver cell susceptibility to infection (22, 70, 83).
It has been also suggested that sporozoite passage through Kupffer cells is not obligatory for infection of hepatocytes. P. berghei sporozoites deficient in SPECT1 and SPECT2 present a large reduction in infectivity of the liver when they are intravenously inoculated into mice (44). In spite of this, these sporozoites are able to infect hepatocytes in vitro with the same effectiveness as they infect the wild strain (70, 135).
Formation of the parasitophorous vacuole requires the participation of the sporozoite's apical organelles, rhoptries and micronemes. In vitro sporozoite incubation with hepatocytes induces the exocytosis of apical organelles containing CS, TRAP, and erythrocyte binding antigen (EBA175) protein. This Ca2+-regulated exocytosis could be induced by Ca2+ ionophores and is independent of sporozoite surface constituent secretion, allowing mobility due to gliding (82).
After the micronemes have discharged their content (including large quantities of TRAP) at the apical extreme, the extracellular domains of these molecules bind to the ligands on the hepatocyte surface, leading to a junction being formed, which precedes invasion (70). Depending on the parasite's maturity within hepatocytes, round exoerythrocytic forms grow and become bigger than the host cell nuclei, generally presenting the CS protein around the periphery (70).
When P. berghei sporozoites invade HepG2 cells in vitro following 24 h of incubation, irradiated and nonirradiated sporozoites become transformed into rounded trophozoites. Forty-eight hours later, most irradiated exoerythrocytic parasites do not present increased size, while nonirradiated ones have increased in size by almost four times. Only 5% of irradiated sporozoites develop into schizonts. Seventy-two hours later, the appearance of merozoites can be observed from just nonirradiated sporozoites (113). These data suggest that irradiated sporozoites are able to invade hepatocytes in vitro and develop into trophozoites but cannot reach maturity. These sporozoites can also induce a more effective immune response than dead sporozoites or sporozoite fractions (113).
Preerythrocyte Stage and Immunity
Infection caused by P. falciparum induces different and complex mechanisms derived from the host's immune response against the different strategies used by the parasite to avoid it. The parasite presents several invasive forms in preerythrocyte and erythrocyte stages, where the latter are those causing the disease's symptomatology. The host's immune system is activated during both phases and T-cell-mediated immune responses, mainly during the preerythrocyte stage. The CD4+-T-cell subset is the most important one for inducing an antibody-mediated immune response during the blood stage, while the CD8+-T-cell subset presents cytotoxic activity against the parasite's hepatic stages (60, 121). The existence of an antigen-presenting dendritic cell subset that is related to the beginning and regulation of the immune cellular response and that is able to produce immune responses during infection caused by the parasite has been recently described. RBC parasitized with P. falciparum are able to suppress in vitro dendritic cell maturation, and it has been suggested that such inhibition may be mediated by the blood-stage parasite (34).
The T lymphocytes play an important role in protection-inducing immunity against the malarial parasite, since it has been found that T-cell subsets can mediate protection or pathogenesis. It has been suggested that in the murine model of infection, antigen presentation by the major histocompatibility complex class II molecule and T-cell costimulation may induce a protection-inducing immune response against the parasite's blood stage. CD4+ and CD25+ T lymphocytes suppress CD4+ and CD8+ cell activation, leading to chronic infections becoming established. It has been reported that these cells suppress T-cell-mediated immunity in mice infected with P. yoelii, as mice with CD25+ cells depleted by an anti-CD25+ monoclonal antibody show protection against P. yoelli infection (34).
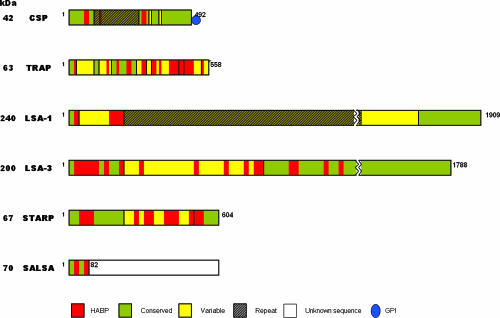
Parasite protein regions that are able to induce an immune cellular response have been reported; some high-binding-activity peptide (HABP) sequences identified in these proteins contain these T epitopes. CS, liver-stage antigen 1 (LSA-1), LSA-3, sporozoite and liver-stage antigen (SALSA), sporozoite threonine- and asparagine-rich protein (STARP), and TRAP possess T epitopes and could be relevant in inducing an effective immune response against the parasite (47, 48, 76, 77, 97, 118).
MOLECULAR ANALYSIS OF P. FALCIPARUM SPOROZOITE LIVER-STAGE PROTEIN AND AMINO ACID SEQUENCE BINDING TO HUMAN HEPATIC CELL LINES
Studying the role of P. falciparum liver-stage proteins in the processes of recognition, binding, and invasion in the vertebrate host is important for a better understanding of the parasite's biology during this stage. These studies will allow the rational development of vaccines leading to controlling malaria caused by P. falciparum during preerythrocyte or liver stages.
In our laboratory, we have developed a methodology for identifying different peptides from these P. falciparum proteins that bind to HepG2 hepatic cells using 15- to 20-amino-acid-long synthetic peptides covering the whole amino acid sequence of the protein being studied (47, 76, 118). Our experimental approach is based on the concept that specific interactions between parasite proteins and cell receptors are of the receptor-ligand type, so HepG2 cell HABPs can be determined with a high degree of accuracy (48, 77, 97). This methodology also allows the calculation of affinity constants, the number of binding sites per cell, Hill constants, and critical residues in the binding being determined. Cross-linking assays have led to the determination that these peptides bind specifically to HepG2 cell membrane proteins. It should be stressed that peptides from some liver-stage proteins also bind to erythrocytes with high binding activity (48, 77, 97).
CS, TRAP, LSA-3, LSA-1, STARP, and SALSA have been studied in our institute, and 61 HepG2 HABP cells have been identified (with 29 of them being conserved) (46, 76, 77, 97, 118); some of them have been modified and tested in immunogenicity studies using Aotus monkeys, assessing their capacity to induce long-lasting antibody titers against P. falciparum sporozoites as determined by immunofluorescence assays (IFAs) and the ability of Western blot assays to recognize some of these recombinant proteins or their fragments. Some of the HABPs identified in different liver-stage proteins such as CS (118), LSA-1 and LSA-3 (47, 48), STARP (77), TRAP (76), and SALSA (97) have been found to be localized in antigenic or immunogenic regions, and some of them have been included in different vaccines against P. falciparum.
Only conserved HABPs have been selected for further analysis due to the tremendous genetic variability of P. falciparum proteins. The conserved HABPs have been identified, and they are synthesized in the polymer form for inoculation into Aotus nancymaae monkeys, a primate species that is highly susceptible to human malaria infection and that has an immune system strikingly similar to that of humans. Our results have shown that conserved sequences possessing high HepG2 cell binding activity are poorly immunogenic. We have previously reported (in studies using merozoite proteins) that modifications of critical residues for binding to cells that are to be invaded (erythrocytes) can render them immunogenic, and a certain number of them become able to protect Aotus nancymaae monkeys against experimental challenge with a highly infective Aotus-adapted P. falciparum strain (36, 99).
Several proteins have been identified as being involved in processes of movement, infection, and development that are relevant for parasite survival. It is possible that surface proteins such as CS, TRAP, SPECT, and SPECT2 are involved in sporozoite movement, interaction with cell receptors, and induction of a protective immune response (44, 63, 64). STARP, LSA-1, LSA-3, SALSA, and secreted protein with altered thrombospondin repeat (SPATR) are some other proteins that have been identified as being involved in parasite survival (28, 70). The molecular identification of their HABPs was thus performed to use them as potential targets for a synthetic multiantigen, multistage, antimalarial vaccine.
CIRCUMSPOROZOITE (CS) PROTEIN
The CS protein is found in all the different Plasmodium parasites and is localized on the sporozoite surface. The genes encoding the CS protein are localized in P. falciparum genome chromosome 3 and have been clearly characterized (4, 23, 24, 86, 90, 100, 123, 128, 136, 137).
This protein is synthesized as a 50- to 70-kDa precursor that is later processed into a 40- to 60-kDa mature surface protein (29, 89). CS protein is an important multifunctional molecule for the parasite, fulfilling different roles (depending on the developmental stage) that are vital for the parasite's development (80). It has been reported that the CS protein is involved in the invasion of a mosquito's salivary glands (112), sporozoite binding to liver cells (24, 43, 101), and inactivating host cell protein synthesis machinery (42).
CS proteins generally present common structural characteristics, with a variable central region composed of tandem repeat amino acid sequences and two highly conserved portions (portions I and II). Portion I, localized in the N terminus, contains the conserved KLKQP motif in different malarial parasites in mammals; this region has been shown to be involved in the invasion of a mosquito's salivary glands (79). Fragments of the protein and peptides containing this motif could inhibit sporozoite invasion of salivary glands (2, 86, 112). This region binds to 35-kDa and 55-kDa proteins present on HepG2-A16 cell membranes as well as to HSPGs; the peptide KLKQP as well as mouse antibodies against this peptide are able to inhibit in vitro P. falciparum sporozoite invasion of HepG2 cells (2, 134).
A peptide from the region's portion I (82DNEKLRKPKHKKLKQADG100), called I-plus, binds saturably to heparin and inhibits CS recombinant protein binding to heparin (4). P. knowlesi and P. yoelii CS proteins present 4,790- to 17,800-times-lower affinity for heparin than P. falciparum CS, suggesting that differences in the protein's amino acid sequences show differences in hepatic cell receptor binding affinity and that this is a mechanism involved in maintaining host specificity for the malaria parasite (101).
The central tandem repeat region is formed in the P. falciparum CS protein by 37 repeat units with the NANP sequence and 4 repeat units with the NVDP sequence (30). These central repeat region sequences (conserved among different Plasmodium species, specifically in P. falciparum) are subjected to selective pressure, since a large number of synonymous polymorphisms have been detected in nucleotide sequences from several P. falciparum isolates (104). This central repeat region is species specific, is immunodominant, has little complexity, and corresponds to around half of the CS protein's amino acid sequences. It has been suggested that the central CS repeat region is involved in initial stages of host cell invasion or that it could perform a structural role in the CS protein (52, 57, 72).
The II-plus region, localized in the CS C-terminal portion, has significant homology with thrombospondin cell (TSR) and properdin domain adhesion (75, 105) as well as with some other sporozoite surface proteins such as TRAP and TRAP-related protein (CTRP) (106, 125).
The II-plus region of this C-terminal portion, consisting of 18 amino acids (EWSXC[S/T]VTCGXG[I/V][Q/R]XRX[K/R]) with limited polymorphism in P. vivax, P. malariae, P. knowlesi, P. berghei, and P. yoelii CS proteins, is involved in binding to HSPGs (23, 105, 115).
CS protein has a canonic inositol glycosylphosphatidylinositol (GPI)-anchoring sequence in its carboxy-terminal segment (93). It has been found that the GPI-anchoring signal motif interferes with this protein's immunogenicity and reduces protection in mice (20). The presence of the GPI-anchoring motif affects complete CS production, cell distribution, antigen processing, and secretion, leading to a loss of antigen presentation (20).
Studies aimed at identifying which amino acids are involved in cellular adhesion have produced contradictory results. Some groups, using sequences derived from thrombospondin, have reached the conclusion that the CSVTCG segment is the motif responsible for cell adhesion activity (126). Work done by other groups with region II plus some peptides has revealed the importance of positively charged residues for CS binding to hepatocytes, suggesting that these residues interact with negatively charged proteoglycan glycosaminoglycan chains, while the CSVTCG sequence does not seem to play any role in this interaction (46). Just like region I, region II is considered to be important because it has been implicated in sporozoite mobility, infection of the vertebrate host, and binding to hepatocytes (115).
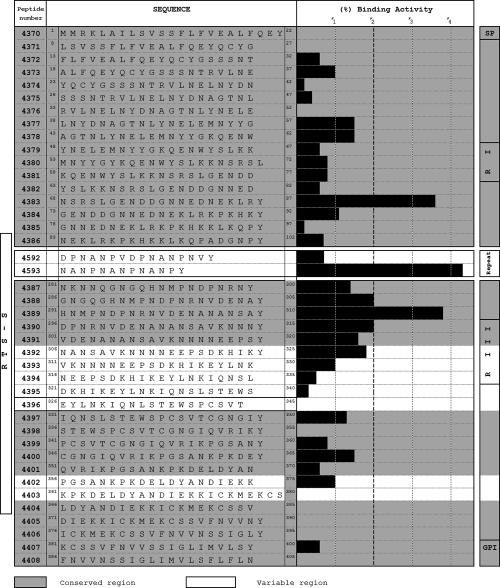
HepG2 cell binding assays (using synthetic peptides) (Fig. 3) carried out in our laboratory have led to the identification of three conserved peptides that present high binding activity (HABPs) to these cells; these peptides are localized in different portions of the protein.
FIG. 3.
HepG2 cell binding activity of five-residue-long overlapping peptides covering the whole CS protein. It can be seen that peptides 4593, 4383, 4388, 4389, and 4390 present high HepG2 cell binding activity. Peptide 4383 is localized in region I; peptide 4593 is localized in the repeat region containing the NANP motif. Peptides 4388, 4389, and 4390 are localized in the protein's following repeat region. (Adapted from reference 118 with permission from Elsevier.)
HABPs 4383 (68NSRSLGENDDGNNDENEKLR87) and 4389 (291HMNPNDPNRNVDENANANSA310) bind to HepG2 cells with high affinity (80 nM and 50 nM, respectively) and specifically bind to a 50-kDa HepG2 cell membrane protein (118). This agrees with what has been suggested regarding the existence of a putative receptor on the human hepatocyte of around 50 kDa involved in the initial binding of P. falciparum sporozoites to target cells (2, 128).
HABP 4383 (68NSRSLGENDDGNNDENEKLR87) is localized in the N-terminal portion (Fig. 3), in the 10-kDa fragment, resulting from CS cleavage by sporozoite cysteine proteases (from the papain family CA clan) when it comes into contact with the hepatic cell membrane when invasion is begun (29). These data suggest that HABP 4383 binding is the first step in activating CS and thereby its functioning later on; it could be the first place where infection could be blocked by CS, since it has been found that CS cleavage is necessary for invading hepatocytes but not for its migration through cells. HABP 4389 (291HMNPNDPNRNVDENANANSA310), localized in portion II following the tandem repeats, is highly conserved, presenting a 50 nM affinity constant in HepG2 cell binding assays. This peptide is found in the RTS-R construct, which has been widely used in preclinical and clinical studies.
The other HABP is a tandem repeat in trimer (NANPNANPNANP) or pentamer form; it is quite interesting that the DPNANPVDPNANPNV sequence does not bind at all to HepG2 cells, making the functional role of this tandem repeat much more obscure. Clinical tests and in vitro assays with recombinant fragments and CS peptides directed at developing a specific immune response against this repeat region have produced frustrating results (25, 54, 55). Attempts to inhibit in vitro sporozoite invasion with peptides containing repeat units have also been unsuccessful (25). It has been suggested that this region acts as a decoy portion for distracting the immune system's attention from more relevant amino acid sequences (HABPs) located in portions I and II.
Other authors have shown that synthetic peptides from P. falciparum CS conserved region II were able to inhibit the recombinant protein's interaction with hepatocytes and that they also inhibited sporozoite invasion of HepG2 cells (24, 103). However, these data should be carefully interpreted, since such receptor-ligand studies do not exclude the possibility of nonspecific interactions, which have always been excluded from our studies (47, 48, 76, 77, 97, 127). P. falciparum peptide P32 (IEQYLKKIKNSISTEWSPCSVTCGNGIQVRIK) and the polyclonal antibodies produced against this peptide have inhibited P. berghei sporozoite invasion of HepG2 cells; mice immunized with this peptide have been protected against a lethal P. berghei sporozoite challenge (27). This peptide presents a high degree of genetic polymorphism; peptides 4396 and 4397 are found in its sequence (underlined) and have a high level of nonspecific HepG2 cell binding activity (Fig. 3).
It has been suggested that region II contains epitopes for T and B cells (27, 78, 102, 108, 124). This has led to phase I, phase II, and phase III preclinical and clinical trials using recombinant region II or RTS with different adjuvants, some of them being extremely potent (RTS-SA), and has led to very short-lived protective immune responses: 29% for only 2 or 3 months (16). This shows that since sporozoite invasion of hepatic cells is a multifactor event, involving multiple parasite proteins with many HABPs, it is not very likely that the results obtained in these vaccination studies could have been due to a protective immune response being induced by just one HABP. A vaccine that is completely effective against the Plasmodium preerythrocyte stage must therefore contain multiple epitopes from many sporozoite and hepatic stage proteins; thus, the HABPs of these molecules represent the best candidates.
Based on previous data and different reports in the literature, the CS protein is considered to be an important candidate in developing vaccines against malaria caused by P. falciparum (3, 87, 122, 130, 131). We therefore consider that the above-mentioned HABPs represent excellent candidates for being included in a subunit-based, multicomponent, multistage, synthetic vaccine against P. falciparum malaria.
THROMBOSPONDIN-RELATED ANONYMOUS PROTEIN (TRAP)
TRAP belongs to a family of functionally homologous proteins involved in parasite mobility due to gliding and cell penetration, suggesting that it could be a mediator for parasite ligand interactions with substrate receptors involved in parasite mobility and with host receptors involved in invasion (69). TRAP is a transmembranal type I protein with two adhesion domains in the extracellular portion: a type A domain (similar to that described as von Willebrand's factor) and a second domain with a motif similar to that of the thrombospondin type I repeat (TSR) (105). A putative TRAP paralogue protein (CTRP) in the Plasmodium ookinete stage has been described, and other putative protein orthologues have been identified in other Apicomplexa such as Toxoplasma (MIC2), Eimeria (Etp100), and Cryptosporidium (TRAPC1) species (69, 125). These proteins have different numbers of type A and TSR domains; however, there is no homology in the primary sequences of the cytoplasmatic tails (69).
TRAP has a cytoplasmatic tail whose primary sequence is not conserved in the different P. falciparum isolates but shows special characteristics: it is acid residue rich (18 to 30%) and contains tryptophan in the penultimate or antepenultimate residue (69). Sporozoites with specific mutations in the penultimate and antepenultimate residues in TRAP's cytoplasmatic tail do not infect a mosquito's salivary glands, rodent livers, and HepG2 cells in vitro. However, these mutations do not cause a loss of gliding but do drastically modify the movement patterns of sporozoites (69).
TRAP has been localized in the parasite's micronemes and apical complex secretor vesicles, which liberate their contents into the parasite's anterior pole (69, 107). Further on, TRAP is liberated onto the substrate during locomotion due to gliding, and during this movement, antibodies against TRAP repeat regions react positively, while antibodies against the cytoplasmatic tail do not react, indicating that TRAP is liberated following protein cleavage (69). The gliding pendular movement presented by these sporozoites could be explained by the sporozoite's ability to transfer TRAP to the posterior pole, making gliding occur in a third part of a circle of the corresponding sporozoite's length, also hampering TRAP liberation and producing an accumulation of this molecule in the posterior pole (69). It is thought that TRAP's cytoplasmatic tail could therefore have a bifunctional nature in the protein's transfer along cortical microfilaments and in liberating TRAP to the posterior pole (69). TRAP is involved in the infection of a mosquito's salivary gland cells as well as mammalian liver cells (120).
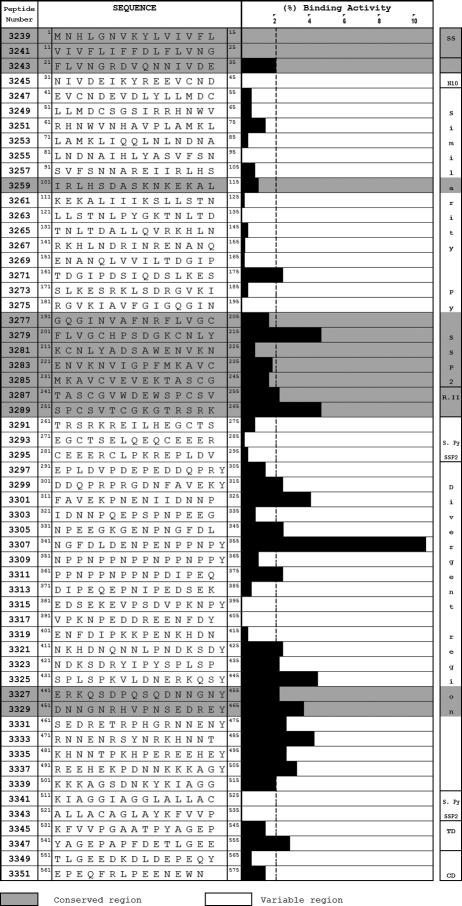
Our HepG2 cell binding assays with 15-mer-long synthetic TRAP peptides (Fig. 4), overlapping each 5 amino acids, led to the finding of conserved HABP 3243 in region I (21FLVNGRDVQNNIVDE35) and equally conserved HABPs 3271 (161TDGIPDSIQDSLKES175) and 3279 (201FLVGCHPSDGKGNLY215) in region II, forming part of this protein's A domain. HABPs 3287 (241TASCGVWDEWSPCSV255) and 3289 (251SPCSVTCGKGTRSRK265) (76), localized in the II-plus region, are equally conserved and contain part of the P. falciparum TRAP CTRP adhesion domains (EWXXCSXTCGXG), analogues of conserved motifs present in the thrombospondin I and II families and M- and F-spondin, Mindin-I and -II, properdin, CS, and CTRP (105, 107, 125) (Fig. 4). Part of this peptide's amino acid sequence is contained in the PT18 epitope (14). This confirms the high specificity (since nonspecific interactions were excluded) and sensitivity of the binding of these motifs to hepatocytes, as described by other authors (85, 106).
FIG. 4.
Peptide sequences of P. falciparum TRAP (from peptides overlapping each five residues) and HepG2 cell binding profiles of synthetic peptides. It can be seen that 21 peptides from TRAP have high HepG2 cell binding activity; a binding region formed by 10 peptides is localized in the C-terminal region. Peptides 3285 and 3287 were found in region II-plus, while peptides 3271 and 3279 were found in the A domain. (Adapted from reference 76 with permission from Blackwell Publishing.)
We found that variable HABP 3299 (301DDQPRPRGDNFAVEKY315) was localized in region III (contained in the divergent region), containing the RGD domain (underlined), which has been involved in interactions with extracellular integrins and glycoproteins (105). Variable HABPs 3299 (301DDQPRPRGDNFAVEK305) and 3301 (311FAVEKPNENIIDNNP325) were also found in region III; these have been described as being antigenic (41) (Fig. 4).
It has been reported that the CHPSDGK sequence contained in HABP 3279 (201FLVGCHPSDGKCNLY215) is recognized by human sera taken from people inhabiting areas where malaria is endemic (26), while conserved HABP 3271 (161TDGIPDSIQDSLKES175) was localized in a region that was reported to have been involved in sporozoite invasion of salivary glands, where the Asp162 residue mutation has been seen to dramatically affect sporozoite invasion of salivary glands (76, 132) (Fig. 4). Variable HABPs 3323 (421NDKSDRYIPYSPLSP435) and 3331 (461SEDRETRPHGRNNEN475) possess sequences (underlined) that are recognized by antibodies that have partially inhibited sporozoite invasion of HepG2 cells in in vitro assays (26).
Highly conserved HABPs 3327 (441ERQKSDPQSQDNNGN455) and 3329 (451DNNGNRHVPNSEDRE465), localized in region IV, the same as HABP 3347 (541YAGEPAPFDETLGEE555) localized in the transmembranal domain including region V, also represent excellent candidates for an antisporozoite vaccine based on subunits from this protein since they are highly conserved. Immunogenicity studies have been carried out using Aotus monkeys with some of these modified HABPs based on the knowledge acquired by using merozoite proteins.
SPOROZOITE THREONINE- AND ASPARAGINE-RICH PROTEIN (STARP)
Sporozoite threonine- and asparagine-rich protein (a molecule that is consistently expressed on sporozoite surface) was identified and cloned for the first time in P. falciparum laboratory strains and field isolates (38) obtained from a broad range of regions where malaria is endemic; it is also present in other Plasmodium species and has a highly conserved structure (40). Immunofluorescence and immunoelectron microscopy assays using immune sera directed against the protein's central and C-terminal regions have revealed that STARP is expressed on THE sporozoite surface during the intrahepatic stage and has also been detected in early ring stages (18, 38). It has been reported that there is a high prevalence of anti-STARP antibodies, mainly in Rp10; specific Rp10 antibodies, purified with the peptide STNNNTKTISTDNNNTKTI, are able to inhibit sporozoite invasion of hepatocytes at low concentrations (91).
A 2.0-kb STARP gene transcription (which has been demonstrated by reverse PCR and Northern blot hybridization) encodes a 604-residue threonine- and asparagine-rich protein with a molecular mass of 78 kDa (38). The protein contains a central repeat domain complex consisting of three repeat regions known as Mosaic (several small degenerated repeats), Rp45 (containing two 45-amino-acid-long units, repeated and identical in sequence), and Rp10 (consisting of 26 10-amino-acid-long repeat units diverging in sequence and size). The central region presents limited variations in size, while N- and C-terminal nonrepeat (NR) sequences do not vary in size and present a low level of polymorphisms. A highly hydrophobic region is localized in the carboxy-terminal extreme (38, 40).
The Rp10 central repeat domain has been the protein portion that has received the most immunological attention. Sera with high immunoglobulin G antibody titers to STARP were obtained from individuals from areas with high and low levels of malaria transmission. African patient serum (exposed to P. falciparum infection) immunoglobulin G, purified by affinity chromatography using synthetic peptides from this region, has been able to inhibit sporozoite invasion in a dose-dependent way by 48% to 90%, depending on the sera and STARP peptide used for the purification (91).
The immunogenicity of four P. falciparum preerythrocyte antigens (STARP being one of them) has been demonstrated by inducing B- and T-cell responses using Aotus lemurinus monkeys immunized with synthetic peptides representing the parasite's native protein segments (92). It has not been possible to obtain a cytotoxic-T-lymphocyte (CTL) response for the STARP Rp10 region using the same strategy in chimpanzees (10).
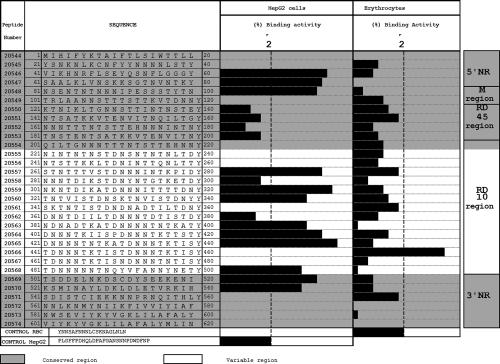
The identification of HepG2 cell HABPs in STARP using receptor-ligand binding assays has led us to recognize 12 peptides that are able to specifically interact with these target cells (77). HABPs 20546 (41VIKIINRFLSEYQSNFLGGGY60), 20547 (61SAALKLVNSKKSGTNVNTKY80), and 20548 (81NSENTNTNNNIPESSSTYTN100) are localized in the N-terminal nonrepeat region, and HABPs 20557, 20559, 20560, 20563, 20564, and 20565 belong to the central domain, in the Rp10 region (Fig. 5). This region's importance lies in the fact that antibodies against the Rp10 region inhibit P. falciparum sporozoite invasion of hepatocytes (91). Conserved HABPs 20569 (501TSDDELNKDSCDYSEEKENI520) and 20570 (521KSMINAYLDKLDLETVRKIH540) are localized in the C-terminal extreme. Cross-linking assays have shown that at least two of these peptides are able to interact with 38-kDa and 44-kDa proteins on the hepatocyte surface (77).
FIG. 5.
Description of different P. falciparum STARP protein regions and the protein's synthetic peptide hepatocyte (HepG2) and human erythrocyte binding activity profiles. Four HepG2 cell binding regions distributed along the protein sequence can be observed. High-binding-activity peptides are localized as follows: peptides 20546 and 20547 were found in the 5′ nonrepeat region; peptide 20548 was found in the M region; peptides 20557, 20559, 20560, 20563, 20564, 20565, and 20568 were found in region 10; and peptide 20570 was found in the 3′ nonrepeat region. (Adapted from reference 77 with permission from Elsevier.)
HABP 20570 (KSMINAYLDKLDLETVRKIH) contains an epitope (underlined) inducing a CTL immune response that is associated with HLA-A2 genetic characteristics. Peptide analogues of HABPs 20546 and 20570 induce high antibody titers against sporozoites in IFAs. All these peptides present saturable binding, having 18 to 219 nM affinity constants. Sera of Aotus monkeys immunized with analogue peptides of HABPs 20546 and 20570 have been shown to recognize a protein on the sporozoite surface by immunofluorescence (77).
STARP's function in the P. falciparum exoerythrocyte cycle in the human host remains unknown; however, all previous evidence supports the idea that the protein may play a role in the sporozoite invasion of hepatocytes and that some epitopes on STARP might be taken into account when a vaccine against this parasite stage is designed.
LIVER-STAGE ANTIGEN 1 (LSA-1)
The LSA-1 protein has a molecular mass of 240 kDa and contains 1,909 amino acids, and the N-and C-terminal regions are conserved in strains Thai, T9/96, and African NF54 (39, 74, 133). This protein contains a 25-amino-acid repeat sequence (EQQSDLEQERRAKEKLQ), spanning residues 154 to 1629, showing an amphipathic α-helix structure (138). The LSA-1 protein is also expressed during early trophozoite and mature parasite stages (39, 74). It is localized around developed schizont pseudocytomers and is associated with flocculent material in the parasitophorous vacuole surrounding exoerythrocytic merozoites. This protein has not been found in the cytoplasm or on the surface of infected hepatocytes; however, it is possible that peptide fragments from this protein may be exported from the parasitophorous vacuole to the hepatocyte surface (35, 39, 74).
LSA-1 possesses potent B and T epitopes in both repeat and nonrepeat regions (39, 58, 66, 74). The protection induced in human volunteers immunized with irradiated P. falciparum sporozoites has been correlated with a proliferative T-cell response from three LSA-1 epitopes (T1, T3, and T5) (73). This protein has been considered to be a candidate for a vaccine against P. falciparum due to the high prevalence of antibodies against LSA-1 among the African population (37, 66).
Some studies have shown that anti-LSA-1 antibodies could inhibit parasite growth by the agglutination of the flocculent mass surrounding recently liberated merozoites and that LSA-1 could block the initial merozoite invasion of erythrocytes (59). It has thus been suggested that this protein is involved in the interaction of the parasite with erythrocytes, contributing to the efficient invasion of erythrocytes by hepatic merozoites (39, 59).
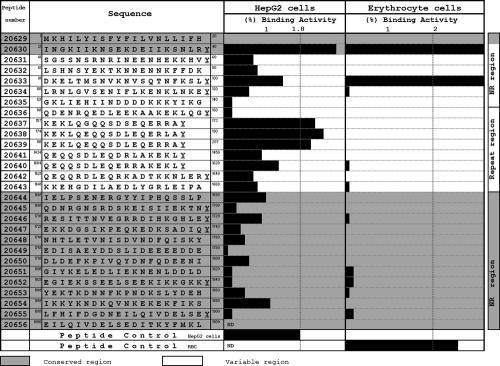
Synthetic HABPs covering the entire protein (Fig. 6) used in hepatocyte and erythrocyte binding assays carried out in our laboratory have shown that peptides 20630 (21INGKIIKNSEKDEIIKSNLRY40) and 20633 (81DKELTMSNVKNVSQTNFKSLY100) bind specifically to both types of cells (47). Peptide 20633 (81DKELTMSNVKNVSQTNFKSLY100) is contained in the T1 epitope (LTMSNVKNVSQTNFKSLRNLGVS), which has been related to gamma interferon production and associated with the absence of parasitemia (66, 67, 74). Hybrid mice immunized with multiple-antigen conjugate containing the T1 epitope present antibodies that recognize sporozoites and the parasite during the hepatic stage; C57BL/10 mouse antibodies immunized with this multiple-antigen conjugate recognize the parasite during the blood stage. This peptide has a high level of homology with the LS1.1 peptide, inducing strong T-cell responses in C57BL/6 mice, and is recognized by sera against the P. berghei liver stage (67). Peptides 20630 and 20633 have also inhibited in vitro P. falciparum invasion of erythrocytes in a concentration-dependent manner (47). Sera from Aotus monkeys immunized with peptide 20630 analogues have inhibited in vitro P. falciparum invasion of erythrocytes (47). These data suggest that peptides 20630 and 20633 could induce antibodies that might also be able to block in vitro P. falciparum invasion of erythrocytes (47) and could therefore be used as components in a synthetic-peptide, multiantigen, multistage, subunit-based vaccine.
FIG. 6.
Hepatic cell (HepG2) and human RBC binding profiles of LSA-1 protein synthetic peptides. Peptide 20630 was identified in the protein's N-terminal region as having high HepG2 cell binding activity. It can also be observed that the protein's repeat region presents high binding activity to these cells; this region is recognized by sera from people inhabiting regions with high levels of malarial transmission. (Adapted from reference 47 with permission from Elsevier.)
LIVER-STAGE ANTIGEN (LSA-3)
The P. falciparum LSA-3 protein (PfLSA-3) was the first preerythrocytic-stage protein to be selected, due to differences in immunological responses among volunteers immunized with irradiated sporozoites who became protected, as opposed to those who were not (32). LSA-3 has a 200-kDa molecular mass and has 1,786 amino acids (1, 32). The protein's amino acid sequence is divided into three nonrepeat regions, called NRA (residues 1 to 221), NRB (residues 819 to 1535), and NRC (residues 1578 to 1786), and three repeat regions, called R1 (residues 222 to 278), R2 (residues 279 to 818), and R3 (residues 1536 to 1577) (32). NRA has a hydrophobic, noncharged, 17-amino-acid-long fragment that is considered to be a signal sequence for subcellular localization in the sporozoite and the liver (1). NRB is highly conserved in the T9/96y parasite clone in the K1 sequence. A restricted epitope associated with HLA-B53, called la90, is localized in this region (1).
R1 represents a series of six tetrapeptides (VEEK/N/S) that are conserved in clones T9/96 and 3D7. R2 contains a series of 75 repeated sequences formed by octapeptides containing the VEESVAEN motif, having two to seven octapeptides depending on the strain. R3 is characterized by the presence of amino acid sequences presenting regularly spaced Val and Ile residues, favoring an α-helical secondary structure in this region. This region is highly conserved in clone 3D7 sequences and among isolates from several regions (http://www.pasteur.fr/parmed/lsa3).
The LSA-3 protein is expressed in sporozoites and is present both in the parasitophorous vacuole and on the periphery of mature hepatic merozoites (32). Antibodies obtained from mice and chimpanzees immunized with LSA-3 protein lipopeptides detect LSA-3 expression on sporozoites and hepatocytes infected with P. falciparum (12, 32). Immune sera from Aotus monkeys inoculated with peptides whose amino acid sequences belong to the LSA-3 protein have recognized the native protein on the surfaces of sporozoites as well as in hepatic schizonts from two distinct strains and in all sporozoites from 30 wild isolates that were developed in vitro in mosquitoes using Thai gametocytes (92).
LSA-3 mRNA has not been detected in the parasite's blood stage, and anti-LSA-3 antibodies do not present cross-reactivity with infected RBC. Nevertheless, antibodies against the glutamine-rich repeat region show cross-reaction with the homologous D260 protein glutamine-rich repeat region, which has been identified in the parasite's intraerythrocyte stages. This protein has also shown homology with a ring-infected erythrocyte surface antigen protein region (32). LSA-3 presents immunological cross-reactivity with Plasmodium yoelii proteins. Mice immunized with PfLSA-3 DNA induce a potent Th1 response and become protected against experimental challenge with P. yoelii (19, 109).
Synthetic peptides and LSA-3 protein recombinant fragments presenting antigenic and immunogenic properties have induced protection in animal models. LSA-3-NRI, LSA-3-NRII-Lp, LSA-3-RE, and LSA-3-CT1-Lp have induced gamma interferon production, while LSA-3-NRI and LSA-3-RE have presented T-cell proliferation in Aotus monkey lymphocytes (92). Chimpanzees immunized with LSA-1 and LSA-3 recombinant fragments have been protected against three challenges by P. falciparum (32).
Thus, LSA-3 is a preerythrocyte antigen that has been considered to be a good candidate for inclusion in a P. falciparum vaccine due to its protection-inducing, antigenic, and immunogenic properties in animal models.
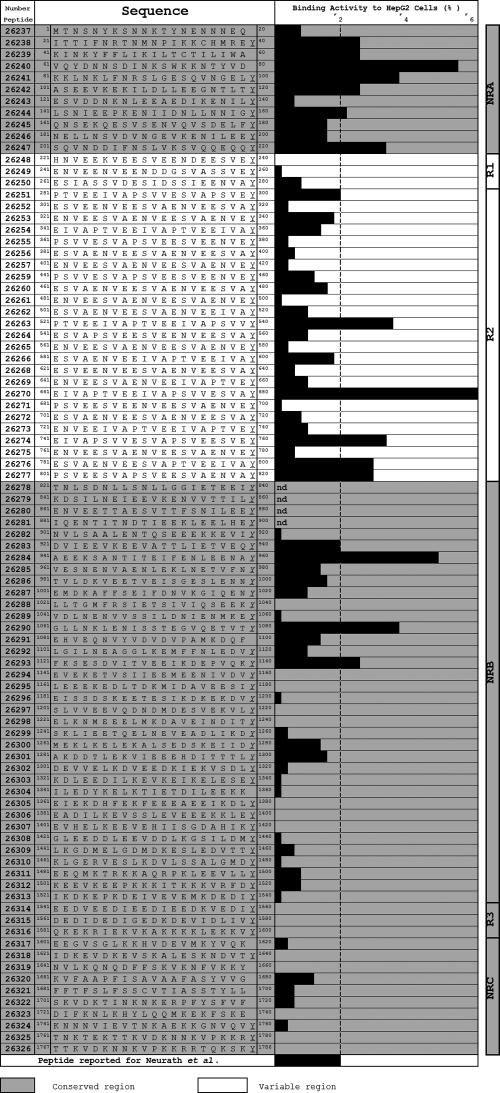
HepG2 cell binding assays with LSA-3 protein-derived peptides showed 17 HABPs localized in repeat and nonrepeat regions specifically binding with high affinity to HepG2 cells (Fig. 6) (48). Some of these HABPs are localized in antigenic and immunogenic protection-inducing regions in animal models (1, 11, 48, 92). Seven conserved HABPs are found in the NRA region: 26238 (21ITTIFNRYNMNPIKKCHMREY40), 26239 (41KINKYFFLIKILTCTILIWA60), 26240 (61VQYDNNSDINKSWKKNTYVD80), 26241 (81KKLNKLFNRSLGESQVNGELY100), 26242 (101ASEEVKEKILDLLEEGNTLTY120), 26244 (141LSNIEEPKENIIDNLLNNIGY160), and 26247 (201SQVNDDIFNSLVKSVQQEQQY220); they have high HepG2 cell binding ability. HABPs 26251 (281PTVEEIVAPSVVESVAPSVEY300), 26263 (521PTVEEIVAPTVEEIVAPSVVY540), 26270 (661EIVAPTVEEIVAPSVVESVAY680), 26274 (741EIVAPSSVVESVAPSVEESVEY760), 26276 (781ESVAENVEESVAPTVEEIVAY800), and 26277 (801PSVEESVAPSVEESVAENVAY820) are found in the R2 region, and HABPs 26283 (921DVIEEVKEEVATTLIETVEQY940), 26284 (941AEEKSANTTITEIFENLEENAY960), 26290 (1061GLLNKLENISSTEGVQETVTY1080), and 26293 (1121FKSESDVITVEEIKDEPVQKY1140) have been identified in the NRB region (Fig. 7). These are similarly conserved and are capable of a high level of binding affinity to these cells. No LSA-3 protein HABP specifically binds to normal human erythrocytes. HABPs belonging to NR regions present affinity constants from 480 nM to 720 nM; interactions between peptides and cells are of the receptor-ligand type, having positive cooperativity. LSA-3 protein HABPs specifically bind to HepG2 cell membrane proteins of 31, 44, and 77 kDa, as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Some of these peptides are localized in antigenic and immunogenic protection-inducing regions in animal models (i.e., LSA-3-CT1, LSA-3-NRI, LSA-3-NRII, and LSA-3-RE) (Fig. 7) (1, 11, 48, 92).
FIG. 7.
LSA-3 protein synthetic peptide hepatic cell (HepG2) binding activity and description of the different regions of the peptides. The figure shows that 17 peptides from LSA-3 present high HepG2 cell binding activity; they are localized in different regions of the protein as follows: a binding region formed by seven peptides (26238 to 26247) was localized in the N-terminal extreme of the NRA region; peptides 26251, 26263, 26270, 26274, 26276, and 26277 were found in region 2; and peptides 26283, 26284, 26290, and 26293 were found in the NRB region. (Adapted from reference 48 with kind permission of Springer Science and Business Medical.)
HABP 26244 (underlined) forms part of the peptide called LSA-3-CT1 (LLSNIEEPKENIIDNLLNNIG), which is considered to be a T epitope. HABP 26247 (SQVNDDIFNSLVKSVQQEQQ), localized in a region called LSA-3-NRII, is considered to be a potent B and T epitope and has protected chimpanzees against P. falciparum infection (11, 12, 32). This suggests that this peptide could be relevant for the interaction between the parasite and the cell and could possibly be considered a good prospect for inducing an immune response that could induce protection against P. falciparum infection during the liver stage (48). The amino acid sequences of HABPs 26251, 26263, 26270, 26274, 26276, and 26277 possess part of the LSA-3-RE peptide, which is considered to be a B epitope.
It has also been found that some LSA-3 protein HABPs possess sequences (considered to be CTL epitopes) presenting HLA-A2 restriction. HABP 26242 (ASEEVKEKILDLLEEGNTLTY) contains the epitope la20 (underlined), and HABP 26290 (GLLNKLENISSTEGVQETVTY) contains the epitope la30 (underlined) (11, 12, 32). Sera from goats immunized with LSA-3 protein polymer peptides recognize P. falciparum intraerythrocyte-stage proteins; this recognition could be due to cross-reactivity with the octapeptide containing the VEESVAEN motif present in the R2 region (48). Some of these conserved HABPs could thus be good candidates for being included in a P. falciparum preerythrocyte synthetic antimalarial vaccine.
SPOROZOITE AND LIVER-STAGE ANTIGEN (SALSA)
Immunofluorescence and immunoelectron microscope studies of sporozoites and liver stages carried out by Bottius et al. (17) have shown that this human Plasmodium falciparum malaria parasite SALSA is a small protein localized on the sporozoite membrane surface and that it continues to be expressed during the liver stage. SALSA is apparently not detected by this type of assay in other Plasmodium species infecting humans (Plasmodium vivax, Plasmodium malariae, and Plasmodium ovale) or in other experimental primate or murine models of infection by Plasmodium cynomolgi, Plasmodium yoelii, or Plasmodium berghei (17).
Analysis of the sequence of the Plasmodium falciparum NF54 strain DG671 clone has revealed that the gene encoding SALSA has a 249-bp open reading frame expressing a 70-kDa polypeptide. The partial sequence of 83 amino acid residues, rich in lysine (19%), glutamic acid (17%), and serine (13%), was deposited in GenBank (accession number AAB36334). It should be stressed that it does not contain methionine, cysteine, and tyrosine. The absence of the long repeat sequences so characteristic of other sporozoite surface proteins (such as CS and TRAP) is also surprising; by contrast, they contain five times the typical pattern of aspartic (D) or glutamic (E) acid residues localized between two lysines (KKDEK, KDDVK, KEEKK, KDDGK, and KVLEK).
Bottius et al., using the PCR DNA amplification technique for the gene encoding SALSA in seven culture-adapted strains (five Asiatic and two African strains) and 16 isolates from Senegal, suggested that the SALSA protein is completely conserved among P. falciparum isolates and pointed out the scarce homology (<30%) between SALSA and other P. falciparum antigens.
Our laboratory has recently reported moderate homology (∼52%) between SALSA and the P. falciparum merozoite surface protein 4, which is known to contain an epidermal growth factor-like domain, and its possible intervention in merozoite invasion of erythrocytes (97).
SALSA protein synthesis begins during the sporozoite stage, possibly during the maturation process in a mosquito's salivary glands; its production increases during hepatic schizogony. According to immunofluorescence assays, this antigen seems to be regularly distributed over the whole sporozoite surface, but it has not been detected on the cytoplasm, according to ultrastructural immunolabeling studies with colloidal gold. It seems to be stored in the granular material from the parasitophorous vacuole membrane in mature hepatic merozoites (17).
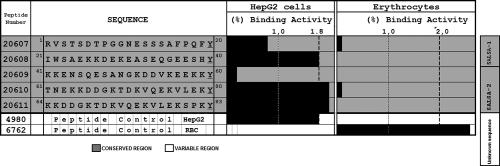
SALSA peptide binding assays have shown that around 50% of the proteins described to date present HepG2 cell binding activity (Fig. 8); HABPs 20608 (21IWSAEKKDEKEASEQGEESHY40), 20610 (61TNEKKDDGKTDKVQEKVLEKY80), and 20611(64KKDDGKTDKVQEKVLEKSPKY83) have been identified. Saturation assays have shown that HABPs 20608 and 20611 present high affinity for HepG2 cells, having nanomolar affinity constants (750 and 600 nM, respectively). Competition assays using glycine analogue peptides have shown that the HABP 20608 C-terminal extreme is responsible for HepG2 cell binding (97). HABPs 20608 and 20610 (underlined) form part of the sequence of a potent B and T epitope (NGKDDVKEEKKTNEKKDDGKTDKVQEKVLEKSPK and SAEKKDEKEASEQGEESHKKENSQESA). Antibodies against these epitopes specifically recognize native protein on the sporozoite surface. It should be kept in mind that these antibodies strongly inhibit sporozoite invasion of hepatic cells (97).
FIG. 8.
Profile of hepatic cell (HepG2) and human RBC binding activity of SALSA protein partial sequence synthetic peptides. It can be seen that three out of the five peptides evaluated presented high HepG2 cell binding activity (i.e., peptides 20608, 20610, and 20611). (Adapted from reference 97 with permission from Elsevier.)
IMMUNOGENICITIES OF CONSERVED HABP ANALOGUES IN AOTUS MONKEYS
Even when antigenicity has been found in some of these conserved HABPs when some sera from individuals living in areas where malaria is highly endemic (mainly in Africa) were used, such antigenicity has been found against recombinant fragments presenting great genetic variability neighboring the conserved HABP. It is probable that such reactivity may be directed against the fragment containing the genetic polymorphism. The fact that the conserved fragments of P. falciparum proteins are poorly immunogenic for any animal species favors such a hypothesis.
Our studies have thus been aimed at converting conserved HABPs (which are poorly immunogenic) into ones that are highly immunogenic for Aotus monkeys (a primate model), which are highly susceptible to developing P. falciparum and P. vivax malaria and whose immune system is very similar to that of humans.
The data regarding polymerized, conserved, native HABPs used for immunizing mice, the same as those used for these monkeys, have confirmed what has been previously observed for P. falciparum merozoite protein-derived conserved HABPs. The absence of immunogenicity of these conserved HABPs has also been observed with sporozoite proteins.
Those residues that are critical for hepatocyte binding (47, 76, 77, 97) (determined by glycine analogue scanning) (Table 1) are thus replaced by amino acids that have a similar mass but different polarity, based on our experience with merozoite HABPs.
TABLE 1.
Critical amino acids in high-activity peptides binding to HepG2 cells from P. faciparum CSP, TRAP, STARP, LSA-1, and SALSA
| Protein | Peptide | Sequencea |
|---|---|---|
| CSP | 4383 | NSRSLGENDDGNNEDNEKLR |
| CSP | 4388 | GNGQGHNMPNDPNRNVDENA |
| CSP | 4389 | HNMPNDPNRNVDENANANSA |
| CSP | 4390 | DPNRNVDENANANSAVKNNN |
| TRAP | 5873 | DLFLVNGRDVQNNIVDEIKYREE |
| TRAP | 5877 | KGENPNGFDLDENPENPPNP |
| TRAP | 5885 | SEDRETRPHGRNNENRSYNRKHNNT |
| TRAP | 6481 | YNRKHNNTPKHPEREEHEKPD |
| TRAP | 5897 | REEHEKPDNNKKKAGSDNKY |
| TRAP | 5891 | GAATPYAGEPAPFDETLGEE |
| STARP | 20546 | VIKHNRFLSEYQSNFLGGGY |
| STARP | 20547 | SAALKLVNSKKSGTNVNTKY |
| STARP | 20548 | NSENTNTNNNIPESSSTYTN |
| STARP | 20569 | TSDDELNKDSSDYSEEKENI |
| STARP | 20570 | KSMINAYLDKLDLETVRKIH |
| LSA-1 | 20630 | INGKIIKNSEKDEIIKSNLRY |
| SALSA | 20608 | IWSAEKKDEKEASEQGEESHY |
Underlined letters are the critical HepG2 cell binding amino acids from each particular peptide sequence.
The results of these changes have shown that determined and specific modifications could convert these HABPs (nonantigenic and nonimmunogenic) into highly immunogenic ones since Aotus monkeys develop high, long-lived antisporozoite antibody titers (determined by IFA) (Fig. 9 and Table 2) and also develop high antibody titers against the parasite's recombinant proteins or its fragments (determined by Western blotting) (data not shown). Conserved HABPs 4383 (derived from CS protein portion I or the N terminus) and 4389 and 4390 (derived from the same protein's portion II or C terminus) become highly immunogenic peptides, since some monkeys develop high and permanent antibody titers against the parasite and their respective recombinant proteins.
FIG. 9.
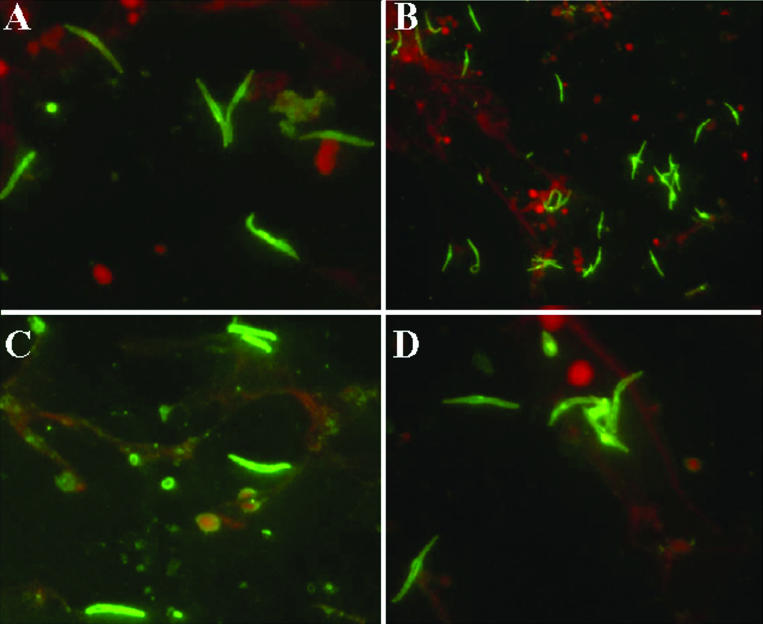
Immunofluorescence assays were carried out with sera from Aotus monkeys that were immunized with peptides from the TRAP protein. (A and B) Sera of monkeys that were immunized with peptide 24246 (HABP 3289 peptide analogue). (C and D) Sera of monkeys that were immunized with peptide 24254 (HABP 3347 peptide analogue).
TABLE 2.
Antibody titers (assessed by IFA against sporozoites) from Aotus monkey sera inmunized with CSP, TRAP, and LSA-1 peptidesa
| Protein | Original peptide | Peptide no.b | Sequence | Titer
|
||||
|---|---|---|---|---|---|---|---|---|
| PI | I20 | II10 | II15 | II30 | ||||
| CS | 4383 | 24258 | CGKKISFSLGEKVIGNNGC | 0 | 0 | 1/640 | ND | 1/320 |
| 4389-4390 | 13516 | CGHNMPNDPIFMSDENANANSAVKNGC | 0 | 0 | ND | 3/320 | 3/640 | |
| 4389-4390 | 13526 | CGHNMPNDPMMMSDENANGMGTVKNGC | 0 | 0 | ND | 1/320 | 1/1,280 | |
| TRAP | 3289 | 24246 | CGSPTSVTVGKGAFSFKREGC | 0 | 1/160 | 3/1,280 | 1/1,280 | 1/1,280 |
| 3347 | 24254 | CGGAATPYSGEPSPFDEVLGEEGC | 0 | 3/640 | 4/640 | 3/640 | 3/1,280 | |
| 3287-3289 | 24242 | CGVWDEWSPVSTAVGMGTRSRKGC | 0 | 5/1,280 | 5/1,280 | 2/1,280 | 2/1,280 | |
| 3277-3279 | 24238 | CGVAFNRFHVGTHPAPGKTNLYGC | 0 | 3/640 | 3/320 | 4/640 | 3/640 | |
| 3285-3287 | 24240 | CGTVEVEMTASTGVWNMWSPGC | 0 | 3/320 | 1/320 | 1/320 | 1/320 | |
| LSA-1 | 20630 | 24282 | CGINGKNIKNAEKPMIIKSNLRGC | 0 | 6/640 | 3/640 | 1/320 | 1/320 |
The analogue peptides were injected on days 0 (I) and 20 (II), and bleeding was carried out at 0 days. (PI) and 20 days after the first immunization (I20) and 10, 15, and 30 days after the second immunization (II10, II15, and II30, respectively). The peptides were tested in groups of five to nine animals (depending on availability). The peptides induced antibodies in Aotus monkeys after the second immunization, which recognized sporozoites by IFA. Underlined letters are the amino acids changed from the original sequence.
Designated by the synthesis group of the Fundacion Instituto de Immunología de Colombia (FIDIC).
The same thing happens with modified HABPs derived from a mixture of TRAP protein HABPs (3277 plus 3279) localized in this protein's A domain and modified HABPs (3285 plus 3287 and 3287 plus 3289) derived from sequences localized in region II-plus containing TRAP protein adhesion domains present in the thrombospondin family.
Something similar happens with the LSA-1 protein-modified conserved HABP 20630 peptide analogue, which binds to both HepG2 cells and erythrocytes. The peptide 20630 analogue (peptide 24282) induces high antibody titers against parasite sporozoites and blood stages, indicating this protein's permanence in both stages (Table 2). This finding and other data suggest that a high, permanent, antibody-mediated immune response could be induced by using modified HABPs from sporozoite proteins. Such antibodies might be able to completely inhibit parasite invasion during these stages (depending on their immunogenetic characteristics) or reduce parasite load, making the effective quantity of sporozoites infecting hepatic cells much lower, thereby reducing the quantity of liberated merozoites and allowing the immune system to mount a greater and better response in a more convenient time. The conserved HABPs mentioned here (involved in hepatic cell invasion, transversal mobility, gliding, and infecting mosquito cells, together with those modifications made to critical binding residues) show that a synthetic multicomponent, multistage vaccine (since it could induce immunity against all of a parasite's development stages) based on subunits is feasible. All this is aimed at reducing the tremendous load imposed by malaria (especially that caused by P. falciparum) on the world's public health.
HABPs found in a protein's conserved regions do not induce antibody production or a protection-inducing response. Such behavior had been observed for HABPs from all the proteins analyzed to date. On the other hand, sequences having low and high levels of specific binding localized in a protein's variable regions do induce high antibody titers in some cases but do not induce protection-inducing immune responses due to their strain specificity. When HABPs localized in conserved regions are finely modified, they become able to induce protection-inducing antibody titers and immune responses in the Aotus model. These modifications are made to those amino acids that are critical for HABP binding to target cells. Such modification is done by changing a critical amino acid for another possessing a similar molecular weight but with a side chain with different physicochemical characteristics. This has led to the obtaining of highly immunogenic sequences that have induced protection in the Aotus model. The advantage of using peptide sequences as vaccine components is that they are pure molecules possessing only the sequence inducing protective immunity, and they are also easy to produce in large quantities and at lower cost.
NOVEL HEPATIC-STAGE PROTEINS RELATED TO INFECTION AND POSSIBLE CANDIDATES FOR DESIGNING VACCINES
We have identified HepG2 cell binding regions for most proteins known to date that have been involved in P. falciparum hepatic-stage binding and invasion. Proteins that have been related to these P. falciparum binding and invasion processes during the hepatic stage and that form the object of our current studies have been described.
These proteins could be considered for designing vaccines against P. falciparum, as the experimental evidence reported by different authors has suggested that these proteins could be involved in the parasite's different infection processes during the hepatic stage. A brief description of the relevance of these proteins in different processes associated with cell infection is given.
Sporozoite Microneme Protein Essential for Cell Transversal (SPECT)
SPECT is a protein that has been involved in sporozoite infection of liver cells. Electron microscopy studies have indicated that this protein is localized on sporozoite micronemes, suggesting that it is possibly involved in sporozoite mobility during invasion. Immunofluorescence studies have shown that SPECT is produced in sporozoites localized in a mosquito's salivary glands (63). In vitro cell invasion assays have shown that sporozoites containing a deletion of the gene encoding SPECT (spect-disrupted parasites) completely lose their ability to pass through Kupffer cells; however, these sporozoites preserve their normal ability to infect hepatocytes, suggesting that SPECT is involved in the ability of sporozoites to pass through cells and that passing through cells and the ability to infect are independent phenomena. Disruption of the gene encoding SPECT does not affect parasite proliferation during the intraerythrocyte stage or a mosquito's development (63).
SPECT2/Plasmodium Perforin-Like Protein 1
SPECT2 is localized on sporozoite micronemes, suggesting that this protein could be involved in invasion (64). Indirect immunofluorescence assays have suggested that this protein's expression is restricted to the salivary gland sporozoite stage. Interrupting the gene encoding the SPECT2 protein in sporozoites does not affect parasite development in the mosquito or the number of sporozoites residing in its stomach and salivary glands (64). This protein contains domains similar to those of the complement system related to perforin (MACPF) membrane attack complex (MAC) characteristic of proteins belonging to the MACPF family, which act by forming pores in the cell membrane, suggesting that this protein could be involved in migration through cells. SPECT2 has been related to the Kupffer cell traversing process before hepatocytes are invaded. Sporozoites intravenously inoculated with the interrupted SPECT2 gene remain in the circulation and do not reach the hepatic parenchyma, suggesting that this protein is necessary for passing through the sinusoidal cell layer (64, 135). Amino acid sequence analysis has revealed that Plasmodium species have five genes related to perforin membrane attack complexes (MACPF) (64). The structure forming the pore is a helix-turn-helix localized in the MACPF mid-domain. These proteins are inserted into the target cell's plasma membrane and form channels for MAC-like polymerization in the target cell membrane to allow the free passage of ions. It has been proposed that MACPF molecules could induce pores to be formed in the host cell membrane and could be involved in parasite invasion of host cells (64). SPECT2 has a highly conserved MACPF domain containing a transmembrane motif, and its amino acid sequence is highly conserved. This suggests that SPECT2 could be involved in transmembrane channel formation in a way similar to those of other molecules related to the MACPF motif.
As happens with SPECT, sporozoites possessing the interrupted SPECT2 gene completely lack the ability for sporozoites to pass through cells and present a great reduction in infectivity in the liver (63, 64), indicating that this protein is essential for sporozoite passage through cells and is involved in passage through the sinusoidal cell layer during infection of the liver. It has been proposed that MACPF molecules could cause pores to become formed in the host cell membrane and could be involved in parasite invasion of host cells (64).
Apical Membrane 3 Antigen/Erythrocyte Binding-Like Protein (MAEBL)
The MAEBL protein has a molecular mass of ∼200 kDa and has 2,055 amino acids; it presents a putative sequence signal in the N-terminal portion that is characterized by having a hydrophobic amino acid rich-region followed by a cleavage site between residues 20 and 21 (50). There are two cysteine-rich regions, called M1 and M2 domains, with high homology with apical membrane antigen-1 domains 1 and 2. There is also a third cysteine-rich domain, which is homologous to the ebl product C-terminal Cys domain close to the transmembrane region and the C-terminal extreme cytoplasmatic domain (50, 96). This protein has been localized on the salivary gland sporozoite surface, in free merozoites, and in late-stage schizonts (15, 50). Antisera against the M1 region and the MAEBL protein cytoplasmatic domain are reactive in sporozoite salivary glands, indicating that this protein's expression occurs in infectious sporozoites (96). Electron microscope and immunofluorescence assays carried out with antibodies against peptides from the P. berghei MAEBL repeat and cytoplasmatic regions have shown a strong positive reaction in the mature sporozoite cytoplasm from oocysts, specifically in the micronemes (71). MAEBL has also been localized in the internal part and on the surface of P. falciparum and Anopheles stephensi sporozoites (96). Parasites that have the disrupted gene encoding P. berghei MAEBL lose their ability to infect salivary glands; however, they maintain their mobility due to gliding, indicating that MAEBL is not essential for in vitro mobility (71). Assays carried out in rats using these sporozoites have suggested that P. berghei MAEBL is not involved in infecting liver cells; however, antisera from rabbits against the M1, M2, and C-terminal Cys domains have presented a high level of inhibition of P. yoelii sporozoite penetration and development in primary hepatocyte cultures (96).
Plasmodium falciparum Secreted Protein with Altered Thrombospondin Repeat (PfSPATR)
Secreted protein with altered thrombospondin repeat (SPATR) consists of 250 amino acids, the first 21 of which represent a putative cleavage signal. Immunofluorescence assays using antibodies against PfSPATR present a positive reaction in all P. falciparum stages (sporozoite and erythrocyte asexual stages and in gametocytes). Immunoelectron microscopy studies have shown that this protein is localized on the sporozoite surface, around the parasite's rhoptries during the erythrocyte asexual stage and, with less intensity, on the infected erythrocyte membrane (28). Western blot assays using sporozoite and parasite blood-stage lysates have shown that antisera against SPATR have detected PfSPATR with a molecular mass of ∼30 kDa during these stages. Sera from people who have been immunized with irradiated sporozoites and clinically immune inhabitants from areas with a high level of P. falciparum malaria transmission recognize the PfSPATR protein expressed on COS-7 cell membrane. SPATR protein binds strongly to HepG2 cells in a concentration-dependent way and strongly inhibits in vitro sporozoite invasion (28).
SUMMARY AND CONCLUSIONS
Developing vaccines against infection caused by P. falciparum could fundamentally lie in (i) identifying proteins that are relevant in infection and inducing an effective immune response against the parasite, (ii) identifying protein regions that are directly involved in mechanisms used by the parasite for infecting cells as well as in developing into new invasive forms, and (iii) evaluating their ability to induce an effective immune response against the parasite, leading to determining whether these regions could be considered possible candidates for vaccines against P. falciparum.
This review presents a compilation of the proteins that have been described to date as being relevant for the parasite's survival and development in the hepatic stage.
HABPs identified for the different proteins described present some important characteristics: (i) some of them are included in or include B and/or T epitopes, (ii) others are localized in target cell binding regions, and (iii) most of them specifically bind to target cell membrane proteins, as has been demonstrated in binding and cross-linking studies. The above-described data indicate that these peptides are localized in the native protein's exposed regions, allowing them to interact with cell receptors. It should also be noted that HABPs belonging to proteins and/or fragments of proteins whose structures have been deduced possess structural characteristics similar to those presented by native proteins, as clearly shown by Puentes et al. (98) and Vera-Bravo et al. (129); this indicates that the interactions of these peptides with cell receptors inducing protection-inducing immune responses are possibly due to interactions depending on structural elements of the peptides in a way similar to what happens in native protein.
Some of the HABPs described for each of these proteins are localized in regions described as being antigenic and immunogenic. Some of these sequences have presented promising results in inducing effective immune responses in the Aotus monkey model, meaning that they can be considered serious candidates for studying and developing multistage, multiantigenic, subunit-based vaccines.
FIG. 10.
Schematic representations of HABPs of the CS, TRAP, STARP, LSA-1, LSA-3, and SALSA sporozoite proteins.
Acknowledgments
This research was supported by the Colombian President's Office and the Instituto Colombiano para el desarrollo de la Ciencia y la Tecnología Francisco José de Caldas (COLCIENCIAS), contract RC-060-2006.
The collaboration of our chemistry and immunology sections is greatly appreciated, as is that of the whole staff at FIDIC. We especially thank Jason Garry for translating and correcting the manuscript.
REFERENCES
- 1.Aidoo, M., A. Lalvani, S. C. Gilbert, J. T. Hu, P. Daubersies, N. Hurt, H. C. Whittle, P. Druihle, and A. V. Hill. 2000. Cytotoxic T-lymphocyte epitopes for HLA-B53 and other HLA types in the malaria vaccine candidate liver-stage antigen 3. Infect. Immun. 68:227-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aley, S. B., M. D. Bates, J. P. Tam, and M. R. Hollingdale. 1986. Synthetic peptides from the circumsporozoite proteins of Plasmodium falciparum and Plasmodium knowlesi recognize the human hepatoma cell line HepG2-A16 in vitro. J. Exp. Med. 164:1915-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alonso, P. L., J. Sacarlal, J. J. Aponte, A. Leach, E. Macete, J. Milman, I. Mandomando, B. Spiessens, C. Guinovart, M. Espasa, Q. Bassat, P. Aide, O. Ofori-Anyinam, M. M. Navia, S. Corachan, M. Ceuppens, M. C. Dubois, M. A. Demoitie, F. Dubovsky, C. Menendez, N. Tornieporth, W. R. Ballou, R. Thompson, and J. Cohen. 2004. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet 364:1411-1420. [DOI] [PubMed] [Google Scholar]
- 4.Ancsin, J. B., and R. Kisilevsky. 2004. A binding site for highly sulfated heparan sulfate is identified in the N terminus of the circumsporozoite protein: significance for malarial sporozoite attachment to hepatocytes. J. Biol. Chem. 279:21824-21832. [DOI] [PubMed] [Google Scholar]
- 5.Baldacci, P., and R. Menard. 2004. The elusive malaria sporozoite in the mammalian host. Mol. Microbiol. 54:298-306. [DOI] [PubMed] [Google Scholar]
- 6.Bannister, L., and G. Mitchell. 2003. The ins, outs and roundabouts of malaria. Trends Parasitol. 19:209-213. [DOI] [PubMed] [Google Scholar]
- 7.Baton, L. A., and L. C. Ranford-Cartwright. 2004. Plasmodium falciparum ookinete invasion of the midgut epithelium of Anopheles stephensi is consistent with the time bomb model. Parasitology 129:663-676. [DOI] [PubMed] [Google Scholar]
- 8.Baton, L. A., and L. C. Ranford-Cartwright. 2005. Do malaria ookinete surface proteins P25 and P28 mediate parasite entry into mosquito midgut epithelial cells? Malar. J. 4:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baton, L. A., and L. C. Ranford-Cartwright. 2005. How do malaria ookinetes cross the mosquito midgut wall? Trends Parasitol. 21:22-28. [DOI] [PubMed] [Google Scholar]
- 10.BenMohamed, L., A. Thomas, and P. Druilhe. 2004. Long-term multiepitopic cytotoxic-T-lymphocyte responses induced in chimpanzees by combinations of Plasmodium falciparum liver-stage peptides and lipopeptides. Infect. Immun. 72:4376-4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benmohamed, L., A. Thomas, M. Bossus, K. Brahimi, J. Wubben, H. Gras-Masse, and P. Druilhe. 2000. High immunogenicity in chimpanzees of peptides and lipopeptides derived from four new Plasmodium falciparum pre-erythrocytic molecules. Vaccine 18:2843-2855. [DOI] [PubMed] [Google Scholar]
- 12.BenMohamed, L., H. Gras-Masse, A. Tartar, P. Daubersies, K. Brahimi, M. Bossus, A. Thomas, and P. Druilhe. 1997. Lipopeptide immunization without adjuvant induces potent and long-lasting B, T helper, and cytotoxic T lymphocyte responses against a malaria liver stage antigen in mice and chimpanzees. Eur. J. Immunol. 27:1242-1253. [DOI] [PubMed] [Google Scholar]
- 13.Bergman, L. W., K. Kaiser, H. Fujioka, I. Coppens, T. M. Daly, S. Fox, K. Matuschewski, V. Nussenzweig, and S. H. Kappe. 2003. Myosin A tail domain interacting protein (MTIP) localizes to the inner membrane complex of Plasmodium sporozoites. J. Cell Sci. 116:39-49. [DOI] [PubMed] [Google Scholar]
- 14.Bharadwaj, A., P. Sharma, S. K. Joshi, B. Singh, and V. S. Chauhan. 1998. Induction of protective immune responses by immunization with linear multiepitope peptides based on conserved sequences from Plasmodium. Infect. Immun. 66:3232-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blair, P. L., S. H. Kappe, J. E. Maciel, B. Balu, and J. H. Adams. 2002. Plasmodium falciparum MAEBL is a unique member of the ebl family. Mol. Biochem. Parasitol. 122:35-44. [DOI] [PubMed] [Google Scholar]
- 16.Bojang, K. A., P. J. Milligan, M. Pinder, L. Vigneron, A. Alloueche, K. E. Kester, W. R. Ballou, D. J. Conway, W. H. Reece, P. Gothard, L. Yamuah, M. Delchambre, G. Voss, B. M. Greenwood, A. Hill, K. P. McAdam, N. Tornieporth, J. D. Cohen, T. Doherty, et al. 2001. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet 358:1927-1934. [DOI] [PubMed] [Google Scholar]
- 17.Bottius, E., L. BenMohamed, K. Brahimi, H. Gras, J. P. Lepers, L. Raharimalala, M. Aikawa, J. Meis, B. Slierendregt, A. Tartar, A. Thomas, and P. Druilhe. 1996. A novel Plasmodium falciparum sporozoite and liver stage antigen (SALSA) defines major B, T helper, and CTL epitopes. J. Immunol. 156:2874-2884. [PubMed] [Google Scholar]
- 18.Bozdech, Z., M. Llinas, B. L. Pulliam, E. D. Wong, J. Zhu, and J. L. DeRisi. 2003. The transcriptome of the intraerythrocytic developmental cycle of Plasmodium falciparum. PLoS Biol. 1:E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brahimi, K., E. Badell, J.-P. Sauzet, L. BenMohamed, P. Daubersies, C. Guérin-Marchand, G. Snounou, and P. Druilhe. 2001. Human antibodies against Plasmodium falciparum liver-stage antigen 3 cross-react with Plasmodium yoelii preerythrocytic-stage epitopes and inhibit sporozoite invasion in vitro and in vivo. Infect. Immun. 69:3845-3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruna-Romero, O., C. D. Rocha, M. Tsuji, and R. T. Gazzinelli. 2004. Enhanced protective immunity against malaria by vaccination with a recombinant adenovirus encoding the circumsporozoite protein of Plasmodium lacking the GPI-anchoring motif. Vaccine 22:3575-3584. [DOI] [PubMed] [Google Scholar]
- 21.Buscaglia, C. A., I. Coppens, W. G. Hol, and V. Nussenzweig. 2003. Sites of interaction between aldolase and thrombospondin-related anonymous protein in Plasmodium. Mol. Biol. Cell 14:4947-4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carrolo, M., S. Giordano, L. Cabrita-Santos, S. Corso, A. M. Vigario, S. Silva, P. Leiriao, D. Carapau, R. Armas-Portela, P. Comoglio, A. Rodriguez, and M. M. Mota. 2003. Hepatocyte growth factor and its receptor are required for malaria infection. Nat. Med. 9:1363-1369. [DOI] [PubMed] [Google Scholar]
- 23.Cerami, C., F. Kwakye-Berko, and V. Nussenzweig. 1992. Binding of malarial circumsporozoite protein to sulfatides [Gal(3-SO4)beta 1-Cer] and cholesterol-3-sulfate and its dependence on disulfide bond formation between cysteines in region II. Mol. Biochem. Parasitol. 54:1-12. [DOI] [PubMed] [Google Scholar]
- 24.Cerami, C., U. Frevert, P. Sinnis, B. Takacs, P. Clavijo, M. J. Santos, and V. Nussenzweig. 1992. The basolateral domain of the hepatocyte plasma membrane bears receptors for the circumsporozoite protein of Plasmodium falciparum sporozoites. Cell 70:1021-1033. [DOI] [PubMed] [Google Scholar]
- 25.Chappel, J. A., W. O. Rogers, S. L. Hoffman, and A. S. Kang. 2004. Molecular dissection of the human antibody response to the structural repeat epitope of Plasmodium falciparum sporozoite from a protected donor. Malar. J. 3:28-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charoenvit, Y., V. Fallarme, W. O. Rogers, J. B. Sacci, Jr., M. Kaur, J. C. Aguiar, L. F. Yuan, G. Corradin, E. Andersen, B. Wizel, R. A. Houghten, A. Oloo, P. De la Vega, and S. L. Hoffman. 1997. Development of two monoclonal antibodies against Plasmodium falciparum sporozoite surface protein 2 and mapping of B-cell epitopes. Infect. Immun. 65:3430-3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chatterjee, S., M. Wery, P. Sharma, and V. S. Chauhan. 1995. A conserved peptide sequence of the Plasmodium falciparum circumsporozoite protein and antipeptide antibodies inhibit Plasmodium berghei sporozoite invasion of Hep-G2 cells and protect immunized mice against P. berghei sporozoite challenge. Infect. Immun. 63:4375-4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chattopadhyay, R., D. Rathore, H. Fujioka, S. Kumar, P. de la Vega, D. Haynes, K. Moch, D. Fryauff, R. Wang, D. J. Carucci, and S. L. Hoffman. 2003. PfSPATR, a Plasmodium falciparum protein containing an altered thrombospondin type I repeat domain is expressed at several stages of the parasite life cycle and is the target of inhibitory antibodies. J. Biol. Chem. 278:25977-25981. [DOI] [PubMed] [Google Scholar]
- 29.Coppi, A., C. Pinzon-Ortiz, C. Hutter, and P. Sinnis. 2005. The Plasmodium circumsporozoite protein is proteolytically processed during cell invasion. J. Exp. Med. 201:27-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dame, J. B., J. L. Williams, T. F. McCutchan, J. L. Weber, R. A. Wirtz, W. T. Hockmeyer, W. L. Maloy, J. D. Haynes, I. Schneider, D. Roberts, G. S. Sanders, E. P. Reddy, C. L. Diggs, and L. H. Miller. 1984. Structure of the gene encoding the immunodominant surface antigen on the sporozoite of the human malaria parasite Plasmodium falciparum. Science 225:593-599. [DOI] [PubMed] [Google Scholar]
- 31.Danforth, D. H., M. Aikawa, A. H. Cochrane, and R. S. Nussenzweig. 1980. Sporozoites of mammalian malaria: attachment to, interiorization and fate within macrophages. J. Protozool. 27:193-202. [DOI] [PubMed] [Google Scholar]
- 32.Daubersies, P., A. W. Thomas, P. Millet, K. Brahimi, J. A. M. Langermans, B. Ollomo, L. BenMohamed, B. Slierendregt, W. Eling, A. Van Belkum, G. Dubreuil, J. F. Meis, C. Guérin-Marchand, S. Cayphas, J. Cohen, H. Gras-Masse, and P. Druilhe. 2000. Protection against Plasmodium falciparum malaria in chimpanzees by immunization with the conserved pre-erythrocytic liver stage antigen 3. Nat. Med. 6:1258-1263. [DOI] [PubMed] [Google Scholar]
- 33.Dhar, R., and N. Kumar. 2003. Role of mosquito salivary glands. Curr. Sci. 89:1308-1313. [Google Scholar]
- 34.Engwerda, C. R., and M. F. Good. 2005. Interactions between malaria parasites and the host immune system. Curr. Opin. Immunol. 17:381-387. [DOI] [PubMed] [Google Scholar]
- 35.Escalante, A. A., A. A. Lal, and F. J. Ayala. 1998. Genetic polymorphism and natural selection in the malaria parasite Plasmodium falciparum. Genetics 149:189-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Espejo, F., M. Cubillos, L. M. Salazar, F. Guzman, M. Urquiza, M. Ocampo, Y. Silva, R. Rodriguez, E. Lioy, and M. E. Patarroyo. 2001. Structure, immunogenicity, and protectivity relationship for the 1585 malarial peptide and its substitution analogues. Angew. Chem. Int. Ed. 40:4654-4657. [DOI] [PubMed] [Google Scholar]
- 37.Ferreira-Da-Cruz, M. F., D. C. Deslandes, J. Oliveira-Ferreira, S. Montenegro-James, A. Tartar, P. Druilhe, and C. T. Daniel-Ribeiro. 1995. Antibody responses to Plasmodium falciparum sporozoite-, liver- and blood-stage synthetic peptides in migrant and autochthonous populations in malaria endemic areas. Parasite 2:23-29. [DOI] [PubMed] [Google Scholar]
- 38.Fidock, D. A., E. Bottius, K. Brahimi, I. I. Moelans, M. Aikawa, R. N. Konings, U. Certa, P. Olafsson, T. Kaidoh, A. Asavanich, C. Guerin-Marchand, and P. Druilhe. 1994. Cloning and characterization of a novel Plasmodium falciparum sporozoite surface antigen, STARP. Mol. Biochem. Parasitol. 64:219-232. [DOI] [PubMed] [Google Scholar]
- 39.Fidock, D. A., H. Gras-Masse, J. P. Lepers, K. Brahimi, L. Benmohamed, S. Mellouk, C. Guerin-Marchand, A. Londono, L. Raharimalala, J. Meis, G. Langsley, C. Roussilhon, A. Tartar, and P. Druilhe. 1994. Plasmodium falciparum liver stage antigen-1 is well conserved and contains potent B and T-cell determinants. J. Immunol. 153:190-204. [PubMed] [Google Scholar]
- 40.Fidock, D. A., S. Sallenave-Sales, J. A. Sherwood, G. S. Gachihi, M. F. Ferreira-da-Cruz, A. W. Thomas, and P. Druilhe. 1994. Conservation of the Plasmodium falciparum sporozoite surface protein gene, STARP, in field isolates and distinct species of Plasmodium. Mol. Biochem. Parasitol. 67:255-267. [DOI] [PubMed] [Google Scholar]
- 41.Flanagan, K. L., T. Mwangi, M. Plebanski, K. Odhiambo, A. Ross, E. Sheu, M. Kortok, B. Lowe, K. Marsh, and A. V. Hill. 2003. Ex vivo interferon-gamma immune response to thrombospondin-related adhesive protein in coastal Kenyans: longevity and risk of Plasmodium falciparum infection. Am. J. Trop. Med. Hyg. 68:421-430. [PubMed] [Google Scholar]
- 42.Frevert, U., M. R. Galinski, F. U. Hugel, N. Allon, H. Schreier, S. Smulevitch, M. Shakibaei, and P. Clavijo. 1998. Malaria circumsporozoite protein inhibits protein synthesis in mammalian cells. EMBO J. 17:3816-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frevert, U., P. Sinnis, C. Cerami, W. Shreffler, B. Takacs, and V. Nussenzweig. 1993. Malaria circumsporozoite protein binds to heparan sulfate proteoglycans associated with the surface membrane of hepatocytes. J. Exp. Med. 177:1287-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frevert, U., S. Engelmann, S. Zougbede, J. Stange, B. Ng, K. Matuschewski, L. Liebes, and H. Yee. 2005. Intravital observation of Plasmodium berghei sporozoite infection of the liver. PLoS Biol. 3:e192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frischknecht, F., P. Baldacci, B. Martin, C. Zimmer, S. Thiberge, J. C. Olivo-Marin, S. L. Shorte, and R. Menard. 2004. Imaging movement of malaria parasites during transmission by Anopheles mosquitoes. Cell. Microbiol. 6:687-694. [DOI] [PubMed] [Google Scholar]
- 46.Gantt, S. M., P. Clavijo, X. Bai, J. D. Esko, and P. Sinnis. 1997. Cell adhesion to a motif shared by the malaria circumsporozoite protein and thrombospondin is mediated by its glycosaminoglycan-binding region and not by CSVTCG. J. Biol. Chem. 272:19205-19213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garcia, J. E., A. Puentes, R. Lopez, R. Vera, J. Suarez, L. Rodriguez, H. Curtidor, M. Ocampo, D. Tovar, M. Forero, A. Bermudez, J. Cortes, M. Urquiza, and M. E. Patarroyo. 2003. Peptides of the liver stage antigen-1 (LSA-1) of Plasmodium falciparum bind to human hepatocytes. Peptides 24:647-657. [DOI] [PubMed] [Google Scholar]
- 48.Garcia, J. E., H. Curtidor, R. Lopez, L. Rodriguez, R. Vera, J. Valbuena, J. Rosas, M. Ocampo, A. Puentes, M. Forero, M. A. Patarroyo, and M. E. Patarroyo. 2004. Liver stage antigen 3 Plasmodium falciparum peptides specifically interacting with HepG2 cells. J. Mol. Med. 82:600-611. [DOI] [PubMed] [Google Scholar]
- 49.Gardner, M. J., N. Hall, E. Fung, O. White, M. Berriman, R. W. Hyman, J. M. Carlton, A. Pain, K. E. Nelson, S. Bowman, I. T. Paulsen, K. James, J. A. Eisen, K. Rutherford, S. L. Salzberg, A. Craig, S. Kyes, M. S. Chan, V. Nene, S. J. Shallom, B. Suh, J. Peterson, S. Angiuoli, M. Pertea, J. Allen, J. Selengut, D. Haft, M. W. Mather, A. B. Vaidya, D. M. Martin, A. H. Fairlamb, M. J. Fraunholz, D. S. Roos, S. A. Ralph, G. I. McFadden, L. M. Cummings, G. M. Subramanian, C. Mungall, J. C. Venter, D. J. Carucci, S. L. Hoffman, C. Newbold, R. W. Davis, C. M. Fraser, and B. Barrell. 2002. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419:498-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghai, M., S. Dutta, T. Hall, D. Freilich, and C. F. Ockenhouse. 2002. Identification, expression, and functional characterization of MAEBL, a sporozoite and asexual blood stage chimeric erythrocyte-binding protein of Plasmodium falciparum. Mol. Biochem. Parasitol. 123:35-45. [DOI] [PubMed] [Google Scholar]
- 51.Ghosh, A., P. E. Ribolla, and M. Jacobs-Lorena. 2001. Targeting Plasmodium ligands on mosquito salivary glands and midgut with a phage display peptide library. Proc. Natl. Acad. Sci. USA 98:13278-13281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Godson, G. N., J. Ellis, P. Svec, D. H. Schlesinger, and V. Nussenzweig. 1983. Identification and chemical synthesis of a tandemly repeated immunogenic region of Plasmodium knowlesi circumsporozoite protein. Nature 305:29-33. [DOI] [PubMed] [Google Scholar]
- 53.Heintzelman, M. B. 2003. Gliding motility: the molecules behind the motion. Curr. Biol. 13:R57-R59. [DOI] [PubMed] [Google Scholar]
- 54.Herrington, D. A., D. F. Clyde, G. Losonsky, M. Cortesia, J. R. Murphy, J. Davis, S. Baqar, A. M. Felix, E. P. Heimer, D. Gillessen, E. Nardin, R. S. Nussenzweig, V. Nussenzweig, M. R. Hollingdale, and M. M. Levine. 1987. Safety and immunogenicity in man of a synthetic peptide malaria vaccine against Plasmodium falciparum sporozoites. Nature 328:257-259. [DOI] [PubMed] [Google Scholar]
- 55.Herrington, D. A., E. H. Nardin, G. Losonsky, I. C. Bathurst, P. J. Barr, M. R. Hollingdale, R. Edelman, and M. M. Levine. 1991. Safety and immunogenicity of a recombinant sporozoite malaria vaccine against Plasmodium vivax. Am. J. Trop. Med. Hyg. 45:695-701. [DOI] [PubMed] [Google Scholar]
- 56.Reference deleted.
- 57.Hoffman, S. L., V. Nussenzweig, J. C. Sadoff, and R. S. Nussenzweig. 1991. Progress toward malaria preerythrocytic vaccines. Science 252:520-521. [DOI] [PubMed] [Google Scholar]
- 58.Hollingdale, M. R., C. J. McCormick, K. G. Heal, A. W. Tailor-Robinson, P. Reeve, R. Boykins, and J. W. Kazura. 1998. Biology of malarial liver stages: implications for vaccine design. Ann. Trop. Med. Parasitol. 92:411-417. [DOI] [PubMed] [Google Scholar]
- 59.Hollingdale, M. R., M. Aikawa, C. T. Atkinson, R. Ballou, G. Chen, J. Li, J. F. Meis, B. Sina, C. Wright, and J. D. Zhu. 1990. Non-Cs pre-erythrocytic protective antigens. Immunol. Lett. 25:71-76. [DOI] [PubMed] [Google Scholar]
- 60.Hviid, L., J. A. Kurtzhals, B. Q. Goka, J. O. Oliver-Commey, F. K. Nkrumah, and T. G. Theander. 1997. Rapid reemergence of T cells into peripheral circulation following treatment of severe and uncomplicated Plasmodium falciparum malaria. Infect. Immun. 65:4090-4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reference deleted.
- 62.Reference deleted.
- 63.Ishino, T., K. Yano, Y. Chinzei, and M. Yuda. 2004. Cell-passage activity is required for the malarial parasite to cross the liver sinusoidal cell layer. PLoS Biol. 2:77-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ishino, T., Y. Chinzei, and Y. Masao. 2005. A Plasmodium sporozoite protein with a membrane attack complex domain is required for breaching the liver sinusoidal cell layer prior to hepatocyte infection. Cell. Microbiol. 7:199-208. [DOI] [PubMed] [Google Scholar]
- 65.James, A. A. 2003. Blocking malaria parasite invasion of mosquito salivary glands. J. Exp. Biol. 206:3817-3821. [DOI] [PubMed] [Google Scholar]
- 66.John, C. C., P. O. Sumba, J. H. Ouma, B. L. Nahlen, C. L. King, and J. W. Kazura. 2000. Cytokine responses to Plasmodium falciparum liver-stage antigen 1 vary in rainy and dry seasons in highland Kenya. Infect. Immun. 68:5198-5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Joshi, S. K., A. Bharadwaj, S. Chatterjee, and V. S. Chauhan. 2000. Analysis of immune responses against T- and B-cell epitopes from Plasmodium falciparum liver-stage antigen 1 in rodent malaria models and malaria-exposed human subjects in India. Infect. Immun. 68:141-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kappe, S. H., K. Kaiser, and K. Matuschewski. 2003. The Plasmodium sporozoite journey: a rite of passage. Trends Parasitol. 19:135-143. [DOI] [PubMed] [Google Scholar]
- 69.Kappe, S., T. Bruderer, S. Gantt, H. Fujioka, V. Nussenzweig, and R. Menard. 1999. Conservation of a gliding motility and cell invasion machinery in apicomplexan parasites. J. Cell Biol. 147:937-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kappe, S. H., C. A. Buscaglia, and V. Nussenzweig. 2004. Plasmodium sporozoite molecular cell biology. Annu. Rev. Cell Dev. Biol. 20:29-59. [DOI] [PubMed] [Google Scholar]
- 71.Kariu, T., M. Yuda, K. Yano, and Y. Chinzei. 2002. MAEBL is essential for malarial sporozoite infection of the mosquito salivary gland. J. Exp. Med. 195:1317-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kobayashi, F., M. Tsuji, and W. P. Weidanz. 2001. In vitro antibody response to the tetrapeptide repeats of Plasmodium falciparum circumsporozoite protein by splenocytes from mice infected with P. yoelii yoelii. J. Vet. Med. Sci. 63:743-749. [DOI] [PubMed] [Google Scholar]
- 73.Krzych, U., J. A. Lyon, T. Jareed, I. Schneider, M. R. Hollingdale, D. M. Gordon, and W. R. Ballou. 1995. T lymphocytes from volunteers immunized with irradiated Plasmodium falciparum sporozoites recognize liver and blood stage malaria antigens. J. Immunol. 155:4072-4077. [PubMed] [Google Scholar]
- 74.Kurtis, J. D., M. R. Hollingdale, A. J. Luty, D. E. Lanar, U. Krzych, and P. E. Duffy. 2001. Pre-erythrocytic immunity to Plasmodium falciparum: the case for an LSA-1 vaccine. Trends Parasitol. 17:219-223. [DOI] [PubMed] [Google Scholar]
- 75.Lawler, J., and R. O. Hynes. 1986. The structure of human thrombospondin, an adhesive glycoprotein with multiple calcium-binding sites and homologies with several different proteins. J. Cell Biol. 103:1635-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.López, R., H. Curtidor, M. Urquiza, J. Garcia, A. Puentes, J. Suarez, M. Ocampo, R. Vera, L. E. Rodriguez, F. Castillo, G. Cifuentes, and M. E. Patarroyo. 2001. Plasmodium falciparum: binding studies of peptide derived from the sporozoite surface protein 2 to Hep G2 cells. J. Pept. Res. 58:285-292. [DOI] [PubMed] [Google Scholar]
- 77.Lopez, R., J. Garcia, A. Puentes, H. Curtidor, M. Ocampo, R. Vera, L. E. Rodriguez, J. Suarez, M. Urquiza, A. L. Rodriguez, C. A. Reyes, C. G. Granados, and M. E. Patarroyo. 2003. Identification of specific Hep G2 cell binding regions in Plasmodium falciparum sporozoite-threonine-asparagine-rich protein (STARP).Vaccine 21:2404-2411. [DOI] [PubMed] [Google Scholar]
- 78.Malik, A., R. Houghten, G. Corradin, S. Buus, J. A. Berzofsky, and S. L. Hoffman. 1995. Identification of a nonameric H-2Kk-restricted CD8+ cytotoxic T lymphocyte epitope on the Plasmodium falciparum circumsporozoite protein. Infect. Immun. 63:1955-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McCutchan, T. F., J. C. Kissinger, M. G. Touray, M. J. Rogers, J. Li, M. Sullivan, E. M. Braga, A. U. Krettli, and L. H. Miller. 1996. Comparison of circumsporozoite proteins from avian and mammalian malarias: biological and phylogenetic implications. Proc. Natl. Acad. Sci. USA 93:11889-11894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Menard, R., A. A. Sultan, C. Cortes, R. Altszuler, M. R. van Dijk, C. J. Janse, A. P. Waters, R. S. Nussenzweig, and V. Nussenzweig. 1997. Circumsporozoite protein is required for development of malaria sporozoites in mosquitoes. Nature 385:336-340. [DOI] [PubMed] [Google Scholar]
- 81.Morrissette, N. S., and L. D. Sibley. 2002. Cytoskeleton of apicomplexan parasites. Microbiol. Mol. Biol. Rev. 66:21-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mota, M. M., and A. Rodriguez. 2002. Invasion of mammalian host cells by Plasmodium sporozoites. BioEssays 24:149-156. [DOI] [PubMed] [Google Scholar]
- 83.Mota, M. M., G. Pradel, J. P. Vanderberg, J. C. Hafalla, U. Frevert, R. S. Nussenzweig, V. Nussenzweig, and A. Rodriguez. 2001. Migration of Plasmodium sporozoites through cells before infection. Science 291:141-144. [DOI] [PubMed] [Google Scholar]
- 84.Mota, M. M., J. C. Hafalla, and A. Rodriguez. 2002. Migration through host cells activates Plasmodium sporozoites for infection. Nat. Med. 8:1318-1322. [DOI] [PubMed] [Google Scholar]
- 85.Muller, H. M., I. Reckmann, M. R. Hollingdale, H. Bujard, K. J. Robson, and A. Crisanti. 1993. Thrombospondin related anonymous protein (TRAP) of Plasmodium falciparum binds specifically to sulfated glycoconjugates and to HepG2 hepatoma cells suggesting a role for this molecule in sporozoite invasion of hepatocytes. EMBO J. 12:2881-2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Myung, J. M., P. Marshall, and P. Sinnis. 2004. The Plasmodium circumsporozoite protein is involved in mosquito salivary gland invasion by sporozoites. Mol. Biochem. Parasitol. 133:53-59. [DOI] [PubMed] [Google Scholar]
- 87.Nardin, E. H., G. A. Oliveira, J. M. Calvo-Calle, K. Wetzel, C. Maier, A. J. Birkett, P. Sarpotdar, M. L. Corado, G. B. Thornton, and A. Schmidt. 2004. Phase I testing of a malaria vaccine composed of hepatitis B virus core particles expressing Plasmodium falciparum circumsporozoite epitopes. Infect. Immun. 72:6519-6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ngo, H. M., M. Yang, and K. A. Joiner. 2004. Are rhoptries in apicomplexan parasites secretory granules or secretory lysosomal granules? Mol. Microbiol. 52:1531-1541. [DOI] [PubMed] [Google Scholar]
- 89.Nussenzweig, V., and R. S. Nussenzweig. 1985. Circumsporozoite proteins of malaria parasites. Cell 42:401-403. [DOI] [PubMed] [Google Scholar]
- 90.Pancake, S. J., G. D. Holt, S. Mellouk, and S. L. Hoffman. 1992. Malaria sporozoites and circumsporozoite proteins bind specifically to sulfated glycoconjugates. J. Cell Biol. 117:1351-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pasquetto, V., D. A. Fidock, H. Gras, E. Badell, W. Eling, W. R. Ballou, J. Belghiti, A. Tartar, and P. Druilhe. 1997. Plasmodium falciparum sporozoite invasion is inhibited by naturally acquired or experimentally induced polyclonal antibodies to the STARP antigen. Eur. J. Immunol. 27:2502-2513. [DOI] [PubMed] [Google Scholar]
- 92.Perlaza, B. L., M. Arévalo-Herrera, K. Brahimi, G. Quintero, J. C. Palomino, H. Gras-Masse, A. Tartar, P. Druilhe, and S. Herrera. 1998. Immunogenicity of four Plasmodium falciparum preerythrocytic antigens in Aotus lemurinus monkeys. Infect. Immun. 66:3423-3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peterson, D. S., Y. Gao, K. Asokan, and J. Gaertig. 2002. The circumsporozoite protein of Plasmodium falciparum is expressed and localised to the cell surface in free-living ciliate Tetrahymena thermophila. Mol. Biochem. Parasitol. 122:119-126. [DOI] [PubMed] [Google Scholar]
- 94.Pimenta, P. F., M. Touray, and L. Miller. 1994. The journey of malaria sporozoites in the mosquito salivary gland. J. Eukaryot. Microbiol. 41:608-624. [DOI] [PubMed] [Google Scholar]
- 95.Pradel, G., and U. Frevert. 2001. Malaria sporozoites actively enter and pass through rat Kupffer cells prior to hepatocyte invasion. Hepatology 33:1154-1165. [DOI] [PubMed] [Google Scholar]
- 96.Preiser, P., L. Renia, N. Singh, B. Balu, W. Jarra, T. Voza, O. Kaneko, P. Blair, M. Torii, I. Landau, and J. H. Adams. 2004. Antibodies against MAEBL ligand domains M1 and M2 inhibit sporozoite development in vitro. Infect. Immun. 72:3604-3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Puentes, A., J. Garcia, R. Vera, R. Lopez, J. Suarez, L. Rodriguez, H. Curtidor, M. Ocampo, D. Tovar, M. Forero, A. Bermudez, J. Cortes, M. Urquiza, and M. E. Patarroyo. 2004. Sporozoite and liver stage antigen Plasmodium falciparum peptides bind specifically to human hepatocytes. Vaccine 22:1150-1156. [DOI] [PubMed] [Google Scholar]
- 98.Puentes, A., M. Ocampo, L. E. Rodriguez, R. Vera, J. Valbuena, H. Curtidor, J. Garcia, R. Lopez, D. Tovar, J. Cortes, Z. Rivera, and M. E. Patarroyo. 2005. Identifying Plasmodium falciparum merozoite surface protein-10 human erythrocyte specific binding regions. Biochimie 87:461-472. [DOI] [PubMed] [Google Scholar]
- 99.Purmova, J., L. M. Salazar, F. Espejo, M. H. Torres, M. Cubillos, E. Torres, Y. Lopez, R. Rodriguez, and M. E. Patarroyo. 2002. NMR structure of Plasmodium falciparum malaria peptide correlates with protective immunity. Biochim. Biophys. Acta 1571:27-33. [DOI] [PubMed] [Google Scholar]
- 100.Rathore, D., J. B. Sacci, P. de la Vega, and T. F. McCutchan. 2002. Binding and invasion of liver cells by Plasmodium falciparum sporozoites. Essential involvement of the amino terminus of circumsporozoite protein. J. Biol. Chem. 277:7092-7098. [DOI] [PubMed] [Google Scholar]
- 101.Rathore, D., S. C. Hrstka, J. B. Sacci, Jr., P. de la Vega, R. J. Linhardt, S. Kumar, and T. F. McCutchan. 2003. Molecular mechanism of host specificity in Plasmodium falciparum infection: role of circumsporozoite protein. J. Biol. Chem. 278:40905-40910. [DOI] [PubMed] [Google Scholar]
- 102.Reece, W. H., M. Pinder, P. K. Gothard, P. Milligan, K. Bojang, T. Doherty, M. Plebanski, P. Akinwunmi, S. Everaere, K. R. Watkins, G. Voss, N. Tornieporth, A. Alloueche, B. M. Greenwood, K. E. Kester, K. P. McAdam, J. Cohen, and A. V. Hill. 2004. A CD4(+) T-cell immune response to a conserved epitope in the circumsporozoite protein correlates with protection from natural Plasmodium falciparum infection and disease. Nat. Med. 10:406-410. [DOI] [PubMed] [Google Scholar]
- 103.Rich, K. A., F. W. George IV, J. L. Law, and W. J. Martin. 1990. Cell-adhesive motif in region II of malarial circumsporozoite protein. Science 249:1574-1577. [DOI] [PubMed] [Google Scholar]
- 104.Rich, S. M., M. U. Ferreira, and F. J. Ayala. 2000. The origin of antigenic diversity in Plasmodium falciparum. Parasitol. Today 16:390-396. [DOI] [PubMed] [Google Scholar]
- 105.Robson, K. J., J. R. Hall, M. W. Jennings, T. J. Harris, K. Marsh, C. I. Newbold, V. E. Tate, and D. J. Weatherall. 1988. A highly conserved amino-acid sequence in thrombospondin, properdin and in proteins from sporozoites and blood stages of a human malaria parasite. Nature 335:79-82. [DOI] [PubMed] [Google Scholar]
- 106.Robson, K. J., U. Frevert, I. Reckmann, G. Cowan, J. Beier, I. G. Scragg, K. Takehara, D. H. Bishop, G. Pradel, R. Sinden, S. Saccheo, H. M. Muller, and A. Crisanti. 1995. Thrombospondin-related adhesive protein (TRAP) of Plasmodium falciparum: expression during sporozoite ontogeny and binding to human hepatocytes. EMBO J. 14:3883-3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rogers, W. O., A. Malik, S. Mellouk, K. Nakamura, M. D. Rogers, A. Szarfman, D. M. Gordon, A. K. Nussler, M. Aikawa, and S. L. Hoffman. 1992. Characterization of Plasmodium falciparum sporozoite surface protein 2. Proc. Natl. Acad. Sci. USA 89:9176-9180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Romero, P. J., J. P. Tam, D. Schlesinger, P. Clavijo, H. Gibson, P. J. Barr, R. S. Nussenzweig, V. Nussenzweig, and F. Zavala. 1988. Multiple T helper cell epitopes of the circumsporozoite protein of Plasmodium berghei. Eur. J. Immunol. 18:1951-1957. [DOI] [PubMed] [Google Scholar]
- 109.Sauzet, J. P., B. L. Perlaza, K. Brahimi, P. Daubersies, and P. Druilhe. 2001. DNA immunization by Plasmodium falciparum liver-stage antigen 3 induces protection against Plasmodium yoelii sporozoite challenge. Infect. Immun. 69:1202-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shin, S. C., J. P. Vanderberg, and J. A. Terzakis. 1982. Direct infection of hepatocytes of Plasmodium berghei. J. Protozool. 29:448-454. [DOI] [PubMed] [Google Scholar]
- 111.Sibley, L. D., S. Hakansson, and V. B. Carruthers. 1998. Gliding motility: an efficient mechanism for cell penetration. Curr. Biol. 8:R12-R14. [DOI] [PubMed] [Google Scholar]
- 112.Sidjanski, S. P., J. P. Vanderberg, and P. Sinnis. 1997. Anopheles stephensi salivary glands bear receptors for region I of the circumsporozoite protein of Plasmodium falciparum. Mol. Biochem. Parasitol. 90:33-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sigler, C., P. Leland, and M. Hollingdale. 1984. In vitro infectivity of irradiated Plasmodium falciparum sporozoites to cultured hepatoma cells. Am. J. Trop. Med. Hyg. 33:544-547. [DOI] [PubMed] [Google Scholar]
- 114.Silvie, O., J. F. Franetich, L. Renia, and D. Mazier. 2004. Malaria sporozoite: migrating for a living. Trends Mol. Med. 10:97-100. [DOI] [PubMed] [Google Scholar]
- 115.Sinnis, P., P. Clavijo, D. Fenyo, B. T. Chait, C. Cerami, and V. Nussenzweig. 1994. Structural and functional properties of region II-plus of the malaria circumsporozoite protein. J. Exp. Med. 180:297-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115a.Sinnis, P., and V. Nussenzweig. 1996. Preventing sporozoite invasion of hepatocytes, p. 15-27. In S. L. Hoffman (ed.), Malaria vaccine development: a multi-immune response approach. ASM Press, Washington, D.C.
- 116.Snow, R. W., C. A. Guerra, A. M. Noor, H. Y. Myint, and S. I. Hay. 2005. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 434:214-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Srinivasan, P., E. G. Abraham, A. K. Ghosh, J. Valenzuela, J. M. Ribeiro, G. Dimopoulos, F. C. Kafatos, J. H. Adams, H. Fujioka, and M. Jacobs-Lorena. 2004. Analysis of the Plasmodium and Anopheles transcriptomes during oocyst differentiation. J. Biol. Chem. 279:5581-5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Suarez, J. E., M. Urquiza, A. Puentes, J. E. Garcia, H. Curtidor, M. Ocampo, R. Lopez, L. E. Rodriguez, R. Vera, M. Cubillos, M. H. Torres, and M. E. Patarroyo. 2001. Plasmodium falciparum circumsporozoite (CS) protein peptides specifically bind to HepG2 cells. Vaccine 19:4487-4495. [DOI] [PubMed] [Google Scholar]
- 119.Sultan, A. A. 1999. Molecular mechanisms of malaria sporozoite motility and invasion of host cells. Int. Microbiol. 2:155-160. [PubMed] [Google Scholar]
- 120.Sultan, A. A., V. Thathy, U. Frevert, K. J. Robson, A. Crisanti, R. S. Nussenzweig, and R. Menard. 1997. TRAP is necessary for gliding motility and infectivity of Plasmodium sporozoites. Cell 90:511-522. [DOI] [PubMed] [Google Scholar]
- 121.Sun, P., R. Schwenk, K. White, J. A. Stoute, J. Cohen, W. R. Ballou, G. Voss, K. E. Kester, D. G. Heppner, and U. Krzych. 2003. Protective immunity induced with malaria vaccine, RTS,S, is linked to Plasmodium falciparum circumsporozoite protein-specific CD4+ and CD8+ T cells producing IFN-gamma. J. Immunol. 171:6961-6967. [DOI] [PubMed] [Google Scholar]
- 122.Tao, D., G. Barba-Spaeth, U. Rai, V. Nussenzweig, C. M. Rice, and R. S. Nussenzweig. 2005. Yellow fever 17D as a vaccine vector for microbial CTL epitopes: protection in a rodent malaria model. J. Exp. Med. 201:201-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tewari, R., R. Spaccapelo, F. Bistoni, A. A. Holder, and A. Crisanti. 2002. Function of region I and II adhesive motifs of Plasmodium falciparum circumsporozoite protein in sporozoite motility and infectivity. J. Biol. Chem. 277:47613-47618. [DOI] [PubMed] [Google Scholar]
- 124.Thomas, B. E., M. Manocha, W. Haq, T. Adak, C. R. Pillai, and D. N. Rao. 2001. Modulation of the humoral response to repeat and non-repeat sequences of the circumsporozoite protein of Plasmodium vivax using novel adjuvant and delivery systems. Ann. Trop. Med. Parasitol. 95:451-472. [DOI] [PubMed] [Google Scholar]
- 125.Trottein, F., T. Triglia, and A. F. Cowman. 1995. Molecular cloning of a gene from Plasmodium falciparum that encodes a protein sharing motifs found in adhesive molecules from mammals and plasmodia. Mol. Biochem. Parasitol. 74:129-141. [DOI] [PubMed] [Google Scholar]
- 126.Tuszynski, G. P., V. L. Rothman, A. H. Deutch, B. K. Hamilton, and J. Eyal. 1992. Biological activities of peptides and peptide analogues derived from common sequences present in thrombospondin, properdin and malarial proteins. J. Cell Biol. 116:209-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Urquiza, M., L. E. Rodriguez, J. E. Suarez, F. Guzman, M. Ocampo, H. Curtidor, C. Segura, E. Trujillo, and M. E. Patarroyo. 1996. Identification of Plasmodium falciparum MSP-1 peptides able to bind to human red blood cells. Parasite Immunol. 18:515-526. [DOI] [PubMed] [Google Scholar]
- 128.Van Pelt, J. F., J. Kleuskens, M. R. Hollingdale, J. P. Verhave, T. Ponnudurai, J. H. Meuwissen, and S. H. Yap. 1991. Identification of plasma membrane proteins involved in the hepatocyte invasion of Plasmodium falciparum sporozoites. Mol. Biochem. Parasitol. 44:225-232. [DOI] [PubMed] [Google Scholar]
- 129.Vera-Bravo, R., M. Ocampo, M. Urquiza, J. E. Garcia, L. E. Rodriguez, A. Puentes, R. Lopez, H. Curtidor, J. E. Suarez, E. Torres, F. Guzman, D. Diaz, J. Cortes, M. M. Bravo, A. L. Combita, O. Orozco, and M. E. Patarroyo. 2003. Human papillomavirus type 16 and 18 L1 protein peptide binding to VERO and HeLa cells inhibits their VLPs binding. Int. J. Cancer 107:416-424. [DOI] [PubMed] [Google Scholar]
- 130.Walther, M., S. Dunachie, S. Keating, J. M. Vuola, T. Berthoud, A. Schmidt, C. Maier, L. Andrews, R. F. Andersen, S. Gilbert, I. Poulton, D. Webster, F. Dubovsky, E. Tierney, P. Sarpotdar, S. Correa, A. Huntcooke, G. Butcher, J. Williams, R. E. Sinden, G. B. Thornton, and A. V. Hill. 2005. Safety, immunogenicity and efficacy of a pre-erythrocytic malaria candidate vaccine, ICC-1132 formulated in Seppic ISA 720. Vaccine 23:857-864. [DOI] [PubMed] [Google Scholar]
- 131.Waters, A. P., M. M. Mota, M. R. van Dijk, and C. J. Janse. 2005. Malaria vaccines: back to the future? Science 307:528-530. [DOI] [PubMed] [Google Scholar]
- 132.Wengelnik, K., R. Spaccapelo, S. Naitza, K. J. Robson, C. J. Janse, F. Bistoni, A. P. Waters, and A. Crisanti. 1999. The A-domain and the thrombospondin-related motif of Plasmodium falciparum TRAP are implicated in the invasion process of mosquito salivary glands. EMBO J. 18:5195-5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yang, C. H., P. Shi, V. Udhayakumar, M. P. Alpers, M. M. Povoa, W. A. Hawley, W. E. Collins, and A. A. Lal. 1995. Sequence variations in the non-repetitive regions of the liver stage-specific antigen-1 (LSA-1) of Plasmodium falciparum from field isolates. Mol. Biochem. Parasitol. 71:291-294. [DOI] [PubMed] [Google Scholar]
- 134.Ying, P., M. Shakibaei, M. S. Patankar, P. Clavijo, R. C. Beavis, G. F. Clark, and U. Frevert. 1997. The malaria circumsporozoite protein: interaction of the conserved regions I and II-plus with heparin-like oligosaccharides in heparan sulfate. Exp. Parasitol. 85:168-182. [DOI] [PubMed] [Google Scholar]
- 135.Yuda, M., and T. Ishino. 2004. Liver invasion by malarial parasites; how do malarial parasites break through the host barrier? Cell. Microbiol. 6:1119-1125. [DOI] [PubMed] [Google Scholar]
- 136.Zavala, F., A. H. Cochrane, E. H. Nardin, R. S. Nussenzweig, and V. Nussenzweig. 1983. Circumsporozoite proteins of malaria parasites contain a single immunodominant region with two or more identical epitopes. J. Exp. Med. 157:1947-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zavala, F., M. R. Hollingdale, A. L. Schwartz, R. S. Nussenzweig, and V. Nussenzweig. 1985. Immunoradiometric assay to measure the in vitro penetration of sporozoites of malaria parasites into hepatoma cells. J. Immunol. 134:1202-1205. [PubMed] [Google Scholar]
- 138.Zhu, J., and M. R. Hollingdale. 1991. Structure of Plasmodium falciparum liver stage antigen 1. Mol. Biochem. Parasitol. 48:223-226. [DOI] [PubMed] [Google Scholar]