Abstract
Intracellular Ca2+ levels rapidly rise following cross-linking of the T-cell receptor (TCR) and function as a critical intracellular second messenger in T-cell activation. It has been relatively under appreciated that K+ channels play an important role in Ca2+ influx into T lymphocytes by helping to maintain a negative membrane potential which provides an electrochemical gradient to drive Ca2+ influx. Here we show that the Ca2+-activated K+ channel, KCa3.1, which is critical for Ca2+ influx in reactivated naive T cells and central memory T cells, requires phosphatidylinositol-3 phosphatase [PI(3)P] for activation and is inhibited by the PI(3)P phosphatase myotubularin-related protein 6 (MTMR6). Moreover, by inhibiting KCa3.1, MTMR6 functions as a negative regulator of Ca2+ influx and proliferation of reactivated human CD4 T cells. These findings point to a new and unexpected role for PI(3)P and the PI(3)P phosphatase MTMR6 in the regulation of Ca2+ influx in activated CD4 T cells and suggest that MTMR6 plays a critical role in setting a minimum threshold for a stimulus to activate a T cell.
Stimulation of the T-cell receptor (TCR) results in the rapid influx of Ca2+, which functions as a critical second messenger in the activation and proliferation of T lymphocytes (24). The increase in cytosolic Ca2+ leads to the activation of the phosphatase calcineurin, resulting in the nuclear import of NFATc and the assembly of NFAT transcription complexes, which mediate transcriptional activation of a number of genes critical for T-cell activation (3, 23). Many studies over the past several years have provided important insights into the mechanisms whereby TCR ligation leads to Ca2+ influx (reviewed in reference 24). Following ligation of the TCR, Ca2+ is released from intracellular stores, which leads to the activation of the Ca2+ release-activated channel at the plasma membrane and the influx of extracellular Ca2+. The activities of K+ channels are also critical for maximal Ca2+ influx into T lymphocytes, and these include a voltage-dependent activated K+ channel, Kv1.3, and a Ca2+-dependent activated channel, KCa3.1 (2, 25). By mediating the efflux of K+, both of these channels function to increase Ca2+ influx by helping to maintain a negative membrane potential which provides an electrochemical gradient to drive Ca2+ influx.
KCa3.1 subunits are components of an intermediate conductance Ca2+-activated K+ channel that are expressed in a variety of cells, including T and B lymphocytes, red blood cells, epithelial cells, and vascular smooth muscle cells (5, 11, 12, 14, 27). While KCa3.1 channels are expressed at low levels in resting naive T cells, KCa3.1 channels are rapidly upregulated following T-cell activation and are required for maximal Ca2+ influx and proliferation during the reactivation of naive T cells (2, 25). In contrast, resting naive T cells express the voltage-dependent K+ channel, Kv1.3, which is required for maximal Ca2+ influx into these cells. KCa3.1 channels are also expressed in central memory T cells, while Kv1.3 channels are expressed in effector memory T cells, where they play a pivotal role in Ca2+ influx and the activation of these cells (25, 26).
It has been appreciated for the past several years that KCa3.1 channels are constitutively bound to calmodulin and channel opening occurs only after Ca2+ binds to calmodulin (10, 15, 29). We recently found that, in addition to Ca2+, KCa3.1 channels also require phosphatidylinositol-3 phosphatase [PI(3)P] for activation and are inhibited by myotubularin-related protein 6 (MTMR6), a PI(3)P phosphatase (19, 20). MTMs are a large family of PI(3)P phosphatases and include 14 members in mammalian cells. MTM1 was first identified as the gene mutated in patients with X-linked congenital muscular dystrophy, and MTMR2 and MTMR13 were found to be mutated in a subset of patients with Charcot Marie tooth syndrome 4B, a peripheral demyelinating neuropathy (13, 22). Thus, while all MTMs function as PI(3)P phosphatases, these findings together with other evidence have indicated that MTMs are not functionally redundant with one another but rather regulate distinct biological processes (4, 13, 22, 30). In this regard, we found that KCa3.1 channels are inhibited specifically by only MTMR6 (19, 20; data not shown). We found that the coil-coiled (CC) domain of KCa3.1 specifically interacts with the CC domain of MTMR6 and have proposed that this interaction functions to localize MTMR6 adjacent to KCa3.1, thereby facilitating the dephosphorylation of PI(3)P in the plasma membrane adjacent to KCa3.1 resulting in the inhibition of KCa3.1. In this report, we sought to determine whether MTMR6, by downregulating KCa3.1 channel activity, functions as a negative regulator of KCa3.1 channels and Ca2+ influx in activated naive CD4 T cells.
MATERIALS AND METHODS
Isolation of CD4 T cells and infection with vesicular stomatitis virus G glycoprotein (VSV-G) pseudotyped human immunodeficiency virus (HIV)-derived lentivirus.
CD4 T cells were isolated from peripheral adult blood buffy coats (NY Blood Center) using the CD4 isolation kit from Miltenyi Biotec according to the manufacturer's protocol. We routinely obtained >95% CD4 T cells, as assessed by fluorescence-activated cell sorter, using this procedure (see the supplemental material).
For lentiviral infection, purified naive T cells were preactivated with anti-CD3 and anti-CD28 for two days, washed, and infected with a VSV-G pseudotyped HIV-derived lentivirus expressing internal ribosome entry site-CD24 that has previously been described (21) either alone (control) or containing wild-type MTMR6 [MTMR6(WT)] or phosphatase-dead MTMR6 [MTMR6(PD)] (20). Infected CD24-positive cells were either directly visualized by staining with fluorescein isothiocyanate-labeled anti-CD24 antibodies or purified using biotin-conjugated anti-CD24 antibodies followed by streptavidin-conjugated magnetic activated cell sorter beads according to the manufacturer's protocol (Miltenyi Biotech). Thirty to 50% of human CD4 T cells were infected using this protocol, and greater then 85% of purified cells were CD24 positive (HSA positive) following purification, as assessed by fluorescence-activated cell sorter.
For small interfering RNA (siRNA) transfection, unstimulated human CD4 T cells were electroporated using AMAXA reagents (Amaxa Bioxystems) according to the manufacturer's protocol. After resting overnight to allow recovery, cells were stimulated for two days with anti-CD3 and anti-CD28 as described above. For Ca2+ flux and proliferation experiments, cells were rested overnight in interleukin-2 prior to restimulation. A pool of siRNAs to human MTMR6 were purchased from Dharmacon. The sense sequences of siRNA oligonucleotides against MTMR6 used in the pool are as follows: 1, GAGAUUGCCAUGAUAUUUAUU; 2, GAACAUGUACCAUCAAUUUUU; 3, UAUCAAAGCUGUUAUGGAUUU; 4, GGAAGUCAAUGGCACUAAAUU.
Patch clamping.
Whole-cell patch clamping was performed on activated CD4 T cells 48 to 72 h after stimulation with anti-CD3 and antiCD28 antibodies as described previously (26) with some modification. Briefly, CD4 T cells were adhered to a laminin-coated coverslip, and patch clamping was performed at room temperature using a pipette solution containing the following (in mM, pH 7.2): K+ aspartate, 147; MgCl2, 2; HEPES, 10; EGTA, 10; CaCl2, 9.85 (10 μM free Ca2+). A bath solution was also used, which contained the following (in mM): Na+ aspartate, 160; KCl, 4.5; CaCl2, 2; MgCl2, 1; HEPES, 5. Patch clamp pipettes had resistances ranging between 4 to 5 MΩ. Current-voltage (IV) relationships were measured using ramp voltage clamp protocols (at 10-s intervals) from a holding potential of −80 mV to −120 mV, followed by ramp depolarization to +60mV of 200-ms duration. To reduce voltage-dependent potassium conductance, 10 μM free Ca2+ was used in the pipette solution (1). The current-voltage relationship was obtained by plotting the current during the depolarizing ramp phase as a function of the corresponding voltage. Membrane currents were filtered (−3 dB at 1 kHz) and digitized at 10 kHz (pClamp 9.2 with Digidata 1200 ADC interface; Axon Instruments). Cell capacitance and pipette series resistances were compensated (usually >80%), and these were obtained using the “membrane test” function of Clampex. Cell capacitance for unstimulated cells was 2 to 2.5 pF, whereas activated cells had a membrane capacitance greater than 8 pF. The whole-cell current density was expressed as nA.
To determine whether phosphatidylinositol 3-kinase (PI3K) inhibition affects channel activity, CD4 T cells were treated with the PI3K inhibitor wortmannin (100 nM) 45 min prior to patch clamping, and the IV relationship assay was performed as described above. To determine whether wortmannin treatment or overexpression of MTMR6 inhibited KCa3.1 by decreasing PI(3)P levels, we determined whether the addition of PI(3)P (100 nM) into the pipette solution during patch clamping restored channel activity. PI(3)P [C41H45Na3O16P2 (C6)] as well as other PIs were purchased from Echelon Biosciences and used according to specifications. All PIs were resuspended in water, flash frozen in liquid nitrogen, and used at a concentration of 100 nM in the pipette solution.
Intracellular Ca2+ activity.
Cells were loaded at 1 × 106 cells/ml with 10 μM Fluo-4 AM ester (Molecular Probes) in RPMI medium for 30 min at room temperature, washed, and resuspended in RPMI. Cells were attached to poly-l-lysine-coated coverslip for 20 min in an RC-20 bath flow chamber (Warner Instrument Corp., Hamden, CT) and analyzed by laser confocal microscopy (Leica Microsystmes, Allendale, New Jersey) equipped with a 488-nm laser using a 63× oil objective. Line-scan images were obtained every 5 s. Background fluorescences obtained from regions containing no cells were digitally subtracted from each image. Data are represented as F/F0, with F representing fluorescence values at different time points and F0 representing cellular fluorescence at time zero. Cells were perfused with the bath solution (composition described previously) in the presence or absence of extracellular calcium and stimulated with 5 μg/ml of anti-CD3 cross-linked with 5 μg/ml of rat anti-mouse IgG.
Quantitative reverse transcription (RT)-PCR.
Total RNA was isolated using Trizol reagent (Invitrogen) and then reverse transcribed using random hexamer primers. Quantitative PCR was then assessed using SYBR green 1 by iCycler iQ (Bio-Rad) using gene-specific primers purchased from QIAGEN.
Proliferation assays.
Human dendritic cells (DCs) were purified and cultured in the presence of granulocyte-macrophage colony-stimulating factor as described previously (16). For proliferation assays, DC were plated together with CD4 T cells in U-bottom 96-well plates at a ration of 10:1 (T cells:DCs) in the presence of various concentrations of staphylococcal enterotoxin B (SEB). Forty-eight hours after stimulation, cells were pulsed with [3H]thymidine, and [3H]thymidine incorporation was assessed as described previously (7).
RESULTS
PI(3)P is required for KCa3.1 channel activity in human CD4 T cells.
To determine whether KCa3.1 channels in CD4 T cells require PI(3)P for activity, human CD4 T cells were stimulated with antibodies to CD3 and CD28 and whole-cell patch clamping was performed 48 h after stimulation in the presence and absence of the PI3K inhibitor wortmannin. As previously reported, KCa3.1 channel activity was markedly upregulated following T-cell activation (compare Fig. 1A and B) as a result of an increase in KCa3.1 expression (see Fig.3Ai) (8). KCa3.1 channels required PI(3)P for activity because channel activity was inhibited more then 90% by wortmannin treatment (Fig. 1C) and inhibition by wortmannin could be completely rescued by including PI(3)P but not other phosphoinositides in the pipette solution during patch clamping (Fig. 1D, E).
FIG. 1.
Endogenous KCa3.1 channel activity in human CD4 T cells requires PI(3)P for activity. Human CD4 T cells were purified from buffy coats by negative selection using the CD4 isolation kit (Miltenyi Biotec) according to the manufacturer's protocol and stimulated for 48 h with antibodies to CD3 and CD28. Whole-cell patch clamping was performed on unstimulated cells (resting) (A) and stimulated T cells in the absence (B) and presence (C) of wortmannin as indicated. (D) Wortmannin-inhibited current was rescued by dialyzing cells with PI(3)P but not by other phosphorylated phosphoinositide (E). (E) Summary data showing TRAM-34-sensitive KCa3.1 current (n = 10 cells). Resting cells predominantly express Kv1.3 current, as determined by its characteristic activation induced by depolarization and inhibition to the specific Kv1.3 inhibitor Shk (27). In contrast, activated CD4 T cells predominantly express KCa3.1, as determined by (i) sensitivity to cytosolic Ca2+, (ii) inhibition by the specific KCa3.0.1 inhibitor TRAM-34, (iii) dependence of the reversal potential on extracellular K, and (iv) voltage independence (27).
FIG. 3.
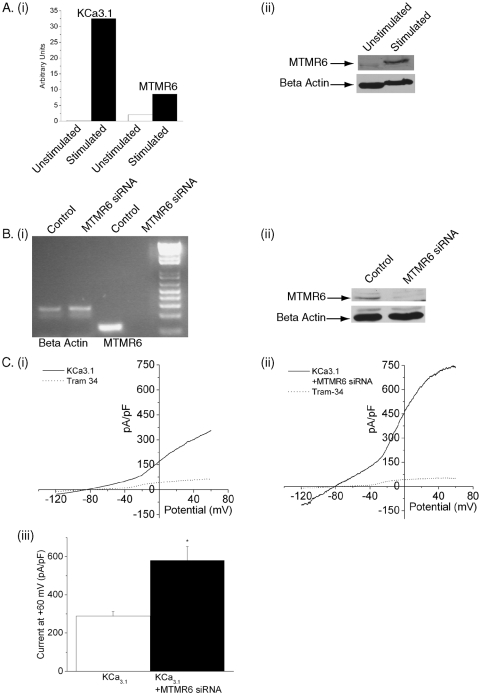
Silencing of MTMR6 in CD4 T cells by siRNA leads to an increase in KCa3.1 channel activity. Purified CD4 T lymphocytes were transfected with a pool of siRNAs to MTMR6 (Dharmacon) or a control siRNA using AMAXA reagents and, after resting overnight, were stimulated with antibodies to CD3 and CD28. Whole-cell patch clamping was performed 48 h after stimulation as described in the legend to Fig. 1. (A) CD4 T cells were either unstimulated or stimulated for 48 h with antibodies to CD3 and CD28. (i) Real time RT-PCR and (ii) Western blotting were performed on total RNA and cell lysates, respectively. The relative amounts of SK4 and MTMR6 in panel i are standardized against beta actin, and an actin control is shown in panel ii to control for protein loading. (B) RT-PCR (i) and Western blotting (ii) of control cells or cells treated with siRNA to MTMR6. An actin control is shown to verify that the quantity of mRNA and protein was similar between samples. (C) Whole-cell patch clamping was performed as described in the legend to Fig. 1. Currents from siRNA control (i) and siRNA MTMR6 (ii) cells are shown. (iii) Summary data of KCa3.1 current from control and siRNA MTMR6 cells are shown. Note the increase in current in MTMR6 siRNA-treated cells (n = 12 to 15) (P < 0.001).
KCa3.1 channel activity is inhibited by MTMR6 in CD4 T cells.
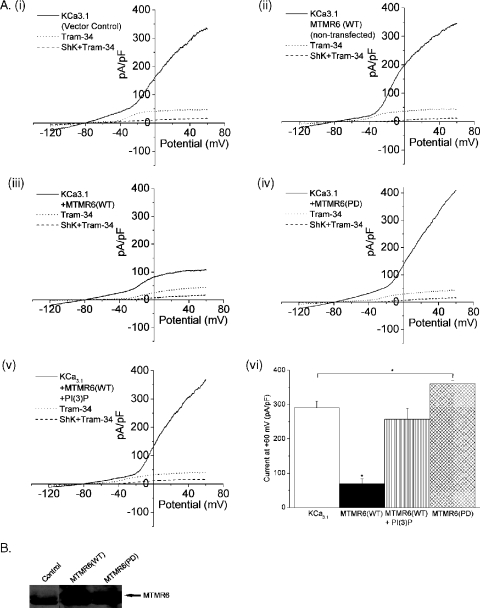
To address whether MTMR6 negatively regulates KCa3.1 channel activity in CD4 T cells, we determined whether overexpression of MTMR6 in CD4 T cells inhibited KCa3.1 channel activity. Similar to wortmannin treatment, overexpression of MTMR6 inhibited KCa3.1 channel activity (Fig. 2Aiii and Avi). Inhibition by MTMR6 was due to the dephosphorylation of PI(3)P because KCa3.1 channel activity in MTMR6-overexpressing CD4 T cells was completely restored by including PI(3)P in the pipette solution during whole-cell patch clamping (Fig. 2Av). In contrast, overexpression of MTMR6(PD) containing a point mutation in the phosphatase domain led to a statistically significant increase in KCa3.1 channel activity (Fig. 2Aiv and Avi), suggesting that MTMR6(PD) functions as a dominant negative and interferes with the function of the endogenous MTMR6 in T cells.
FIG. 2.
MTMR6 inhibits KCa3.1 in CD4 T cells. Purified human naive CD4 T cells were preactivated with anti-CD3 and anti-28, washed, and infected with a VSV-G-pseudotyped HIV-derived lentivirus expressing internal ribosome entry site-HSA(CD24) that has been described previously (21) either alone (control) or containing Flag-tagged MTMR6(WT) or MTMR6(PD). Infected HSA-positive cells were then purified using biotin-conjugated goat anti-CD24 antibodies followed by streptavidin-conjugated magnetic activated cell sorter beads (Miltenyi Biotec). After resting overnight, whole-cell patch clamping was performed as described in the legend to Fig. 1 on stimulated T cells. (A) Membrane currents from (i) control, (iii) MTMR6-infected, and (iv) MTMR6(PD)-infected cells. (v) MTMR6-inhibited current was rescued by dialyzing MTMR6-infected cells with PI(3)P. (vi) Summary data of TRAM-34 inhibitable KCa3.1 current from different interventions as indicated (n = 10 to 12) (P < 0.05). (ii) Additional control of noninfected cells (CD24 negative) from cells infected with MTMR6(WT) lentivirus. (B) Western blot of CD4 T-cell lysates probed with polyclonal antibodies to MTMR6.
In agreement with the idea that MTMR6 functions in CD4 T cells, we found that MTMR6 mRNA, as well as the mRNA of several other MTMs, is expressed in human CD4 T cells and that MTMR6 mRNA and protein are upregulated following activation with anti-CD3 and CD28 antibodies (Fig. 3Ai and ii). To verify that endogenous MTMR6 plays a critical role in negatively regulating KCa3.1 channel activity, naive CD4 T cells were transfected with siRNA to MTMR6 and, after resting overnight, were stimulated for 48 h with antibodies to CD3 and CD28. MTMR6 mRNA and protein were silenced more then 90% using a pool of siRNAs (Dharmacon) to MTMR6 (Fig. 3B). Moreover, silencing of MTMR6 was associated with about a twofold increase in KCa3.1 channel activity compared to cells transfected with an siRNA control (Fig. 3C). In addition, the same results were obtained by silencing MTMR6 using an siRNA that targeted a different sequence in MTMR6, indicating that the “off target” effects of the siRNAs do not account for the increase in KCa3.1 channel activity (see the supplemental material).
MTMR6 negatively regulates Ca2+ influx in CD4 T cells.
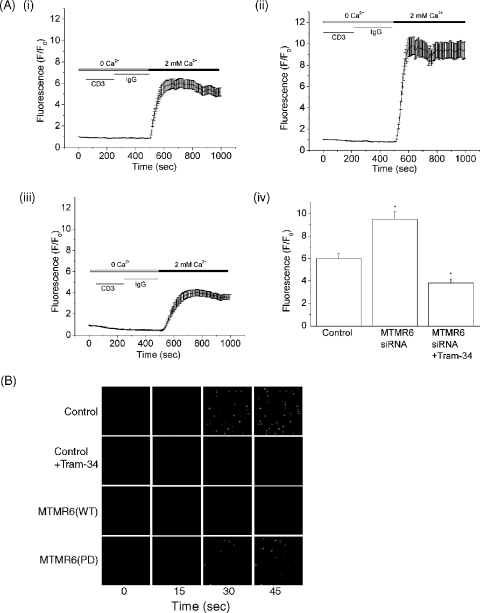
We next determined whether the increase in KCa3.1 channel activity in MTMR6 siRNA-treated cells was also associated with an increase in Ca2+ influx. siRNA-treated cells were stimulated for 48 h with antibodies to CD3 and CD28 and, after resting overnight, were loaded with Fluo-4 AM (10 μM), and Ca2+ influx was determined by confocal microscopy following cross-linking with anti-CD3 antibodies. Ca2+ influx in anti-CD3 reactivated CD4 T cells was about twofold higher in MTMR6 siRNA-treated CD4 T cells than in control siRNA-treated cells (compare Fig. 4Ai and ii). In addition, overexpression of MTMR6(WT) in CD4 T cells not only resulted in a decrease in KCa3.1 channel activity (Fig. 2) but also inhibited Ca2+ influx in response to cross-linking CD3, whereas overexpression of MTMR6(PD) did not (Fig. 4B). Thus, these findings when taken together indicate that MTMR6 functions to downregulate KCa3.1 channel activity, which subsequently leads to the inhibition of Ca2+ influx in reactivated CD4 T cells.
FIG. 4.
Silencing of MTMR6 in CD4 T cells by siRNA leads to an increase in Ca2+ influx. Purified CD4 T cells were transfected with siRNA to MTMR6 as described in the legend to Fig. 3. Cells were then stimulated for 48 h with antibodies to CD3 and CD28 and, after resting overnight, were loaded with Fluo4-AM (10 μM). Ca2+ influx was determined by confocal microscopy at 488 nm with images taken every 5 s after cross-linking with anti-CD3 antibodies (5 μg/ml) as previously described (6). Average values from 80 to 100 cells are shown for each series. Ca2+ influx was determined in control cells (Ai), siRNA MTMR6 cells (Aii), and siRNA MTMR6 cells treated with 1 μM TRAM-34 for 15 min (Aiii). (Aiv) Bar graph showing fluorescence values from panels Ai, Aii, and Aiii at 700 s. (B) Ca2+ influx was determined in CD4 T cells overexpressing MTMR6(WT) or MTMR6(PD) following cross-linking with anti-CD3 antibodies as described for panel A. Overexpression of MTMR6(WT) and treatment of control cells with TRAM-34 (Cont/TRAM-34) inhibits Ca2+ influx.
MTMR6 negatively regulates proliferation of human CD4 T cells.
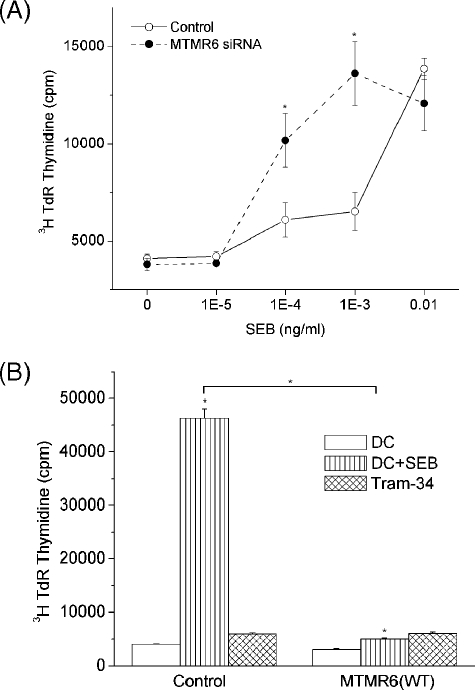
To address whether the change in Ca2+ influx also led to changes in the proliferation of these cells, MTMR6 siRNA-treated CD4 T cells or CD4 T cells overexpressing MTMR6(WT) were stimulated for 48 h as described above, and after resting overnight, proliferation was assessed by [3H]thymidine incorporation after stimulating cells with human DCs in the presence of various concentration SEB as previously described (16). We found that proliferation mirrored the response to Ca2+ influx. MTMR6 siRNA interference-treated CD4 T cells exhibited a shift to the left in the concentration of SEB that was required to stimulate T-cell proliferation (Fig. 5A). We found that SEB stimulated the proliferation of MTMR6 siRNA-treated cells at a concentration that was 10- to 100-fold lower then that required to stimulate proliferation in control treated cells. In contrast to siRNA-treated cells, overexpression of MTMR6 led to marked suppression of proliferation, which mirrored the inhibition seen when cells were treated with the KCa3.1 inhibitor Tram34 (Fig. 5B).
FIG. 5.
MTMR6 negatively regulates proliferation of CD4 T cells. (A) Purified CD4 T cells were treated as described in the legend to Fig. 4 and, after resting overnight, were plated in 96-well plates with human DC that were activated for 24 h with lipopolysaccharide (100 ng/ml) in a ratio of 10:1 (30,000 CD4 T cells:3,000 DC) in the presence of various concentrations of SEB as described previously (16). Twenty-four hours following stimulation, cells were pulsed for 8 h with [3H]thymidine (3H TdR), and [3H]thymidine incorporation was assessed by scintillation counting (7). (B) Proliferation of CD4 T cells was determined in cells overexpressing MTMR6 using a similar protocol to that described for panel A.
DISCUSSION
The findings reported here indicate for the first time that PI(3)P is required for the activation of the intermediate conductance K+ channel, KCa3.1, in CD4 T cells and that the PI(3)P phosphatase MTMR6 specifically downregulates KCa3.1 by decreasing PI(3)P levels. Moreover, by inhibiting KCa3.1, MTMR6 functions to limit Ca2+ influx and the subsequent proliferation of reactivated CD4 T cells. The finding that these data are supported by experiments in which MTMR6 is silenced by RNA interference as well as in experiments in which MTMR6 is overexpressed proves unequivocally that at least one function of MTMR6 is to downregulate KCa3.1 channel activity in CD4 T cells.
We still do not know how the inhibition of the KCa3.1 channel by MTMR6 is regulated in activated CD4 T cells. One possibility is that MTMR6 functions constitutively to tonically inhibit the KCa3.1 channel and thereby sets a threshold for a stimulus to activate a T cell. This function of MTMR6 would then prevent small changes in Ca2+ influx resulting from nonspecific stimulation of the T-cell receptor to provide a sustained Ca2+ signal that would then lead to T-cell activation. This hypothesis is consistent with our data showing that silencing of MTMR6 led to activation of CD4 T cells at a 10-fold lower concentration of antigen than for control cells. This hypothesis is also reinforced by the finding that MTMR6 expression is upregulated along with KCa3.1 channel expression following T-cell activation and that MTMR6 and the KCa3.1 channel are constitutively associated via an interaction between their respective CC domains; the interaction between the CC domains of MTMR6 and the KCa3.1 channel is required to localize MTMR6 to the plasma membrane where it can then dephosphorylate PI(3)P and inhibit the KCa3.1 channel.
Previous studies have shown that PI3K play important roles in the activation of T lymphocytes (9, 17). For the most part, these studies have focused on the role of the class 1 PI3K in which a p85-p110 heterodimer is activated following the recruitment of p85 to phosphotyrosine-containing proteins, such as CD28 and TcR-interacting molecule, leading to the generation of PI(3, 4)P2 and PI(3,4,5)P3. PI(3, 4)P2 and PI(3,4,5)P3 in turn function as second messengers to activate a number of different signaling pathways which include the activation of PDK1 and AKT, the tec family kinase Itk, and a number of guanine nucleotide exchange factors (reviewed in references 9 and 17). Our finding that KCa3.1 activation and Ca2+ influx in activated T cells requires PI(3)P identifies a new role for 3-phosphorylated phosphoinositides in T-cell activation. Moreover, because PI(3)P is primarily generated by the class III PI3K (28), these findings suggest that the class III PI3K plays an important and unexpected role in Ca2+ influx in CD4 T cells, although we cannot rule out the possibility that PI(3)P is generated via the dephosphorylation of PI(3, 4)P2 and PI(3,4,5)P.
It is now known that KCa3.1 channel expression as well as the expression of other ion channels varies between different T-cell subsets and changes dramatically following T-cell activation (23). It is likely that the different combinations of these ion channels and differences in their mode of regulation contribute to the specific functions of different T-cell subsets by providing a means to uniquely regulate T-cell activation in various T-cell populations. With respect to KCa3.1, it has recently been shown that treatment of mice with TRAM-34, a specific inhibitor of KCa3.1, protects mice from developing experimental autoimmune encephalomyelitis by specifically inhibiting the production of inflammatory cytokine by activated T cells invading the spinal cord without affecting basal cytokine production in splenocytes (18). This finding suggests that antigen-specific activated T cells that upregulate KCa3.1 in vivo are uniquely dependent upon KCa3.1 for cytokine production. Future studies will determine whether MTMR6 plays a role in regulating KCa3.1 channel activity and cytokine production using in vivo models, such as experimental autoimmune encephalomyelitis, and to determine whether altered regulation of MTMR6 contributes to disease phenotype.
Supplementary Material
Acknowledgments
This work was supported by NIH grant DK49207.
We thank K. George Chandy (University of California, Irvine) and Heike Wulff (University of California, Davis) for Tram34 and helpful discussions.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Bregestovski, P., A. Redkozubov, and A. Alexeev. 1986. Elevation of intracellular calcium reduces voltage-dependent potassium conductance in human T cells. Nature 319:776-778. [DOI] [PubMed] [Google Scholar]
- 2.Cahalan, M. D., H. Wulff, and K. G. Chandy. 2001. Molecular properties and physiological roles of ion channels in the immune system. J. Clin. Immunol. 21:235-252. [DOI] [PubMed] [Google Scholar]
- 3.Crabtree, G. R., and E. N. Olson. 2002. NFAT signaling: choreographing the social lives of cells. Cell 109(Suppl.):S67-S79. [DOI] [PubMed] [Google Scholar]
- 4.Dang, H., Z. Li, E. Y. Skolnik, and H. Fares. 2004. Disease-related myotubularins function in endocytic traffic in Caenorhabditis elegans. Mol. Biol. Cell 15:189-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fanger, C. M., H. Rauer, A. L. Neben, M. J. Miller, H. Wulff, J. C. Rosa, C. R. Ganellin, K. G. Chandy, and M. D. Cahalan. 2001. Calcium-activated potassium channels sustain calcium signaling in T lymphocytes. Selective blockers and manipulated channel expression levels. J. Biol. Chem. 276:12249-12256. [DOI] [PubMed] [Google Scholar]
- 6.Feske, S., J. Giltnane, R. Dolmetsch, L. M. Staudt, and A. Rao. 2001. Gene regulation mediated by calcium signals in T lymphocytes. Nat. Immunol. 2:316-324. [DOI] [PubMed] [Google Scholar]
- 7.Fournier, E., S. J. Isakoff, K. Ko, C. J. Cardinale, G. G. Inghirami, Z. Li, M. A. de Lafaille, and E. Y. Skolnik. 2003. The B cell SH2/PH domain-containing adaptor Bam32/DAPP1 is required for T cell-independent II antigen responses. Curr. Biol. 13:1858-1866. [DOI] [PubMed] [Google Scholar]
- 8.Ghanshani, S., H. Wulff, M. J. Miller, H. Rohm, A. Neben, G. A. Gutman, M. D. Cahalan, and K. G. Chandy. 2000. Up-regulation of the IKCa1 potassium channel during T-cell activation. Molecular mechanism and functional consequences. J. Biol. Chem. 275:37137-37149. [DOI] [PubMed] [Google Scholar]
- 9.Kane, L. P., and A. Weiss. 2003. The PI-3 kinase/Akt pathway and T cell activation: pleiotropic pathways downstream of PIP3. Immunol. Rev. 192:7-20. [DOI] [PubMed] [Google Scholar]
- 10.Keen, J. E., R. Khawaled, D. L. Farrens, T. Neelands, A. Rivard, C. T. Bond, A. Janowsky, B. Fakler, J. P. Adelman, and J. Maylie. 1999. Domains responsible for constitutive and Ca(2+)-dependent interactions between calmodulin and small conductance Ca(2+)-activated potassium channels. J. Neurosci. 19:8830-8838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khanna, R., M. C. Chang, W. J. Joiner, L. K. Kaczmarek, and L. C. Schlichter. 1999. hSK4/hIK1, a calmodulin-binding KCa channel in human T lymphocytes. Roles in proliferation and volume regulation. J. Biol. Chem. 274:14838-14849. [DOI] [PubMed] [Google Scholar]
- 12.Kohler, R., H. Wulff, I. Eichler, M. Kneifel, D. Neumann, A. Knorr, I. Grgic, D. Kampfe, H. Si, J. Wibawa, R. Real, K. Borner, S. Brakemeier, H. D. Orzechowski, H. P. Reusch, M. Paul, K. G. Chandy, and J. Hoyer. 2003. Blockade of the intermediate-conductance calcium-activated potassium channel as a new therapeutic strategy for restenosis. Circulation 108:1119-1125. [DOI] [PubMed] [Google Scholar]
- 13.Laporte, J., F. Bedez, A. Bolino, and J. L. Mandel. 2003. Myotubularins, a large disease-associated family of cooperating catalytically active and inactive phosphoinositides phosphatases. Hum. Mol. Genet. 12(Spec. no. 2):R285—R292. [DOI] [PubMed] [Google Scholar]
- 14.Maher, A. D., and P. W. Kuchel. 2003. The Gardos channel: a review of the Ca2+-activated K+ channel in human erythrocytes. Int. J. Biochem. Cell Biol. 35:1182-1197. [DOI] [PubMed] [Google Scholar]
- 15.Maylie, J., C. T. Bond, P. S. Herson, W. S. Lee, and J. P. Adelman. 2004. Small conductance Ca2+-activated K+ channels and calmodulin. J. Physiol. 554:255-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Motsinger, A., A. Azimzadeh, A. K. Stanic, R. P. Johnson, L. Van Kaer, S. Joyce, and D. Unutmaz. 2003. Identification and simian immunodeficiency virus infection of CD1d-restricted macaque natural killer T cells. J. Virol. 77:8153-8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okkenhaug, K., A. Bilancio, J. L. Emery, and B. Vanhaesebroeck. 2004. Phosphoinositide 3-kinase in T cell activation and survival. Biochem. Soc. Trans. 32:332-335. [DOI] [PubMed] [Google Scholar]
- 18.Reich, E. P., L. Cui, L. Yang, C. Pugliese-Sivo, A. Golovko, M. Petro, G. Vassileva, I. Chu, A. A. Nomeir, L. K. Zhang, X. Liang, J. A. Kozlowski, S. K. Narula, P. J. Zavodny, and C. C. Chou. 2005. Blocking ion channel KCNN4 alleviates the symptoms of experimental autoimmune encephalomyelitis in mice. Eur. J. Immunol. 35:1027-1036. [DOI] [PubMed] [Google Scholar]
- 19.Srivastava, S., P. Choudhury, Z. Li, G. Liu, V. Nadkarni, K. Ko, W. A. Coetzee, and E. Y. Skolnik. 2006. Phosphatidylinositol 3-phosphate indirectly activates KCa3.1 via 14 amino acids in the carboxy terminus of KCa3.1. Mol. Biol. Cell 17:146-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srivastava, S., Z. Li, L. Lin, G. Liu, K. Ko, W. A. Coetzee, and E. Y. Skolnik. 2005. The phosphatidylinositol 3-phosphate phosphatase myotubularin-related protein 6 (MTMR6) is a negative regulator of the Ca2+-activated K+ channel KCa3.1. Mol. Cell. Biol. 25:3630-3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sundrud, M. S., S. M. Grill, D. Ni, K. Nagata, S. S. Alkan, A. Subramaniam, and D. Unutmaz. 2003. Genetic reprogramming of primary human T cells reveals functional plasticity in Th cell differentiation. J. Immunol. 171:3542-3549. [DOI] [PubMed] [Google Scholar]
- 22.Taylor, G. S., and J. E. Dixon. 2003. PTEN and myotubularins: families of phosphoinositide phosphatases. Methods Enzymol. 366:43-56. [DOI] [PubMed] [Google Scholar]
- 23.Winslow, M. M., and G. R. Crabtree. 2005. Immunology. Decoding calcium signaling. Science 307:56-57. [DOI] [PubMed] [Google Scholar]
- 24.Winslow, M. M., J. R. Neilson, and G. R. Crabtree. 2003. Calcium signalling in lymphocytes. Curr. Opin. Immunol. 15:299-307. [DOI] [PubMed] [Google Scholar]
- 25.Wulff, H., C. Beeton, and K. G. Chandy. 2003. Potassium channels as therapeutic targets for autoimmune disorders. Curr. Opin. Drug Discov. Devel. 6:640-647. [PubMed] [Google Scholar]
- 26.Wulff, H., P. A. Calabresi, R. Allie, S. Yun, M. Pennington, C. Beeton, and K. G. Chandy. 2003. The voltage-gated Kv1.3 K(+) channel in effector memory T cells as new target for MS. J. Clin. Investig. 111:1703-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wulff, H., H. G. Knaus, M. Pennington, and K. G. Chandy. 2004. K+ channel expression during B cell differentiation: implications for immunomodulation and autoimmunity. J. Immunol. 173:776-786. [DOI] [PubMed] [Google Scholar]
- 28.Wymann, M. P., and R. Marone. 2005. Phosphoinositide 3-kinase in disease: timing, location, and scaffolding. Curr. Opin. Cell Biol. 17:141-149. [DOI] [PubMed] [Google Scholar]
- 29.Xia, X. M., B. Fakler, A. Rivard, G. Wayman, T. Johnson-Pais, J. E. Keen, T. Ishii, B. Hirschberg, C. T. Bond, S. Lutsenko, J. Maylie, and J. P. Adelman. 1998. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature 395:503-507. [DOI] [PubMed] [Google Scholar]
- 30.Xue, Y., H. Fares, B. Grant, Z. Li, A. M. Rose, S. G. Clark, and E. Y. Skolnik. 2003. Genetic analysis of the myotubularin family of phosphatases in Caenorhabditis elegans. J. Biol. Chem. 278:34380-34386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.