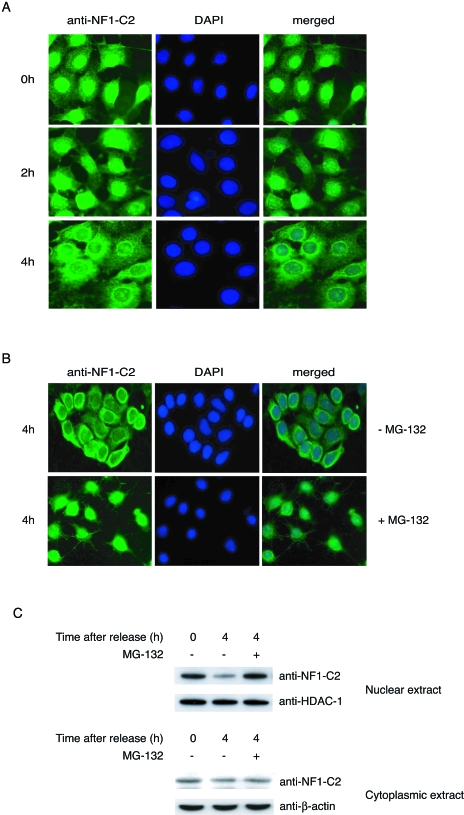
FIG. 2.
NF1-C2 is degraded by the proteasome in vivo in mammary epithelial cells. (A) HC11 cells grown on small object glasses were arrested at the G1/S border by a double thymidine block. The object glasses containing cells were taken out after 0, 2, and 4 h after release in fresh medium. The subcellular localization of NF1-C2 was then analyzed by immunohistochemistry using the NF1-C2-specific antibody (1:100 dilution). After being incubated with the secondary antibody, the cells were stained with fluorescein isothiocyanate. Nuclei were visualized with DAPI (4′,6′- diamidino-2-phenylindole). (B) The double-thymidine-blocked cells were treated or not treated with MG-132 for 4 h as indicated. NF1-C2 proteins were visualized as described above. (C) Nuclear and cytoplasmic extracts were prepared from double-thymidine-blocked cells treated with (+) or without (−) MG-132 for 4 h and subjected to Western blot analysis. The filter containing the nuclear extracts was first incubated with the NF1-C2-specific antibody and then stripped and reincubated with an HDAC-1 antibody. The filter containing the cytoplasmic extracts was first incubated with the NF1-C2-specific antibody and then stripped and reincubated with a β-actin antibody. The HDAC-1 and β-actin blots served as loading controls.