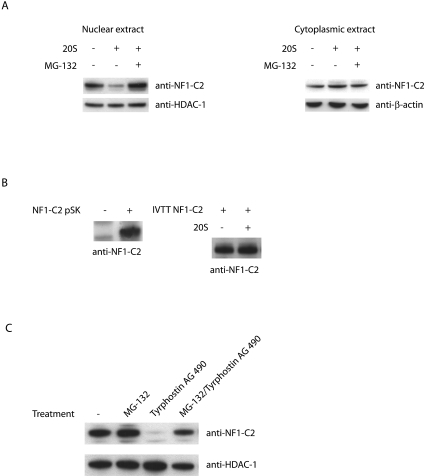
FIG. 6.
Interaction between Jak2 and tyrosine-phosphorylated NF1-C2 prevents NF1-C2 association with and subsequent degradation by the proteasome in vivo. (A) Western blot analysis of subcellular extracts incubated at 37°C for 50 min in the absence (−) or presence (+) of purified 20S proteasome. Where indicated, the proteasome inhibitor MG-132 was added. The blot was incubated with the anti-NF1-C2-specific antibody. HDAC-1 and β-actin were used as loading controls. (B) Western blot on NF1-C2 protein generated with the in vitro transcription/translation (IVTT) TNT-coupled reticulocyte lysate system (left panel). The right panel shows a Western blot analysis of the in vitro transcription/translation NF1-C2 protein treated with or without 20S as indicated. (C) Nuclear proteins were prepared from HC11 cells treated with MG-132 and/or tyrphostin AG490 as indicated and subjected to Western blot analysis. The filter was first incubated with anti-NF1-C2 antibody and then stripped and reincubated with anti-HDAC-1 antibody.