FIG. 1.
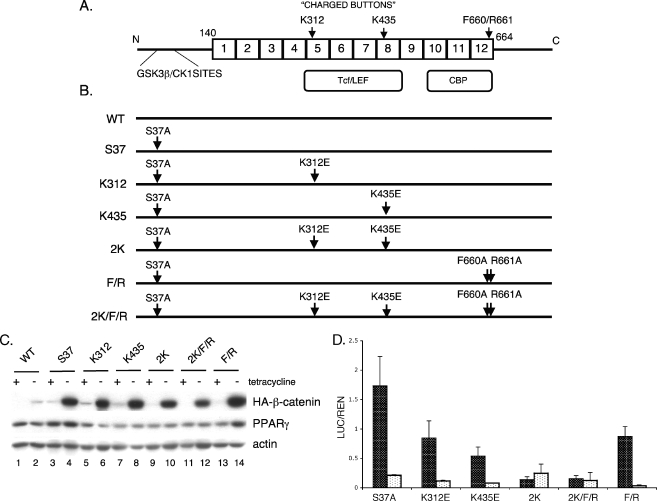
The charged buttons (K312 and K435) of β-catenin are required to activate the canonical Wnt signaling. (A) Structure of β-catenin: GSK3β/CK1 sites are located in the N-terminal domain followed by 12 armadillo repeats (from amino acids [aa] 140 to 664). TCF/LEF and CBP binding domains span from repeats 5 to 8 and 10 to 12, respectively. Two charged buttons (K312 in repeat 5 and K435 in repeat 8) and F660/R661 (in repeat 12) are indicated. (B) Schematic representation of β-catenin mutants. S37 represents a mutant with serine 37 mutated to alanine (S37A). All of the other mutations as indicated were generated within the parental S37A β-catenin molecule (i.e., K312 represents lysine 312 mutated to glutamic acid [K312E]). (C) Expression of the ectopic mutant β-catenins. The stable cell lines expressing the WT or different mutated S37A β-catenins as well as PPARγ were cultured in the presence or absence of tetracycline for 5 days until confluent. Total cellular proteins were analyzed by Western blotting for HA-tagged β-catenin, PPARγ, and actin (loading control). (D) TCF/LEF reporter gene assay. The stable cell lines were cultured in the absence of tetracycline for 3 days, at which stage they were transfected with TOPFLASH (black) or FOPFLASH (white) firefly reporter plasmids along with Renilla luciferase plasmid for an additional 2 days. Then, the luciferase assay was performed as detailed in Materials and Methods. The same experiment was repeated at least three times. The final values (the ratio of luciferase to Renilla [LUC/REN]) and standard deviation (error bars) were calculated based on all repeats.