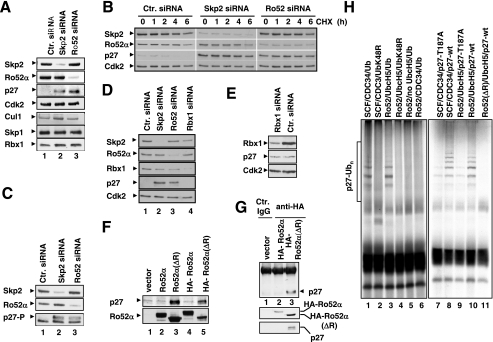
FIG. 5.
Downregulation of Ro52 stabilizes p27. (A) HeLa cells were transfected with siRNAs corresponding to either nonrelevant mRNA (control siRNA) (lane 1), Skp2 mRNA (lane 2), or Ro52 mRNA (lane 3) and processed for immunoblotting with antibodies against indicated proteins. (B) The silencing of Skp2 and Ro52 was performed as described for panel A, and then cycloheximide (CHX) was added for the indicated times and lysates were processed for immunoblotting with antibodies against indicated proteins. (C) HeLa cells were transfected with indicated siRNAs and analyzed by immunoblotting using antibodies against Skp2, Ro52, and the Thr187-phosphorylated form of p27. (D and E) HeLa cells were transfected with indicated siRNAs and processed for immunoblotting for the specified proteins. (F) HeLa cells were transfected with empty vector (lane 1) or expression plasmids encoding untagged Ro52α(wt) (lane 2), untagged Ro52α(ΔR) (lane 3), HA-Ro52α(wt) (lane 4), or HA-Ro52α(ΔR) (lane 5) and processed for immunoblotting for p27 and Ro52. (G) Aliquots of lysates of HeLa cells transfected either with empty vector (lane 1) or with vectors to produce HA-Ro52α(wt) (lane 2) or HA-Ro52α(ΔR) (lane 3) were either subjected to immunoprecipitation with control mouse IgG (lane 1) or anti-HA 12CA5 (lanes 2 and 3) antibody, followed by immunoblotting with rabbit anti-p27 antibody (upper panel), or directly processed for immunoblotting using anti-HA MAb HA11 (middle panel) or anti-p27 antibody (lower panel). (H) Autoradiograms of 35S-labeled p27(wt) (lanes 1 through 6, 8, 10, and 11) or p27(T187A) (lanes 7 and 9) after in vitro ubiquitination by anti-HA 12CA5 immunoprecipitates derived from HeLa cells that were transfected to produce HA-Ro52α, MT-Skp2, and MT-Cul1 (labeled with Ro52) or SCFSkp2 assembled in Sf9 cells (labeled with SCF). ctr., control.