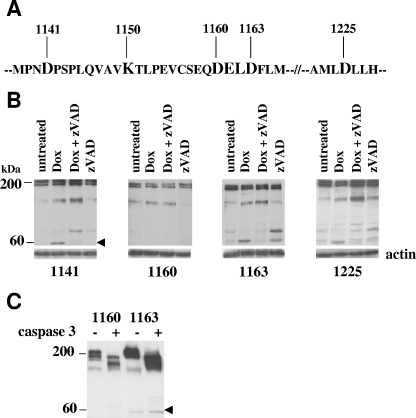
FIG. 4.
The caspase cleavage site of ALK is D1160. (A) Portion of the ALK intracellular sequence showing the caspase-3 consensus-like cleavage site (DELD) and the position of aspartic acids chosen for experimental D_N mutation, as well as the ATP-binding site (K1150). (B) Jurkat cells were permanently transfected with cDNAs coding for ALK mutants displaying a D_N mutation at position 1141, 1160, 1163, or 1225 and cloned by limiting dilution. One representative cell clone is shown for each mutant. Cells were treated with 2 μM doxorubicin for 24 h in the presence or absence of 10 μM zVAD-fmk or left untreated. The arrowhead indicates the 60-kDa ALK fragment, absent from the ALK D1160N mutant-expressing cells. The filters were stripped and reprobed using an antiactin MAb to assess comparable loading. (C) Total protein cell lysates from untreated Jurkat cells expressing the 1160 and 1163 D_N ALK mutants were incubated in vitro with purified activated caspase-3 for 30 min at 37°C and analyzed by Western blotting using the ALKc MAb. The arrowhead indicates the 60-kDa ALK fragment. Lysates from equivalent numbers of cells were loaded onto a 7.5% SDS-PAGE gel.