Abstract
Members of the B7 family of cosignaling molecules regulate T-cell proliferation and effector functions by engaging cognate receptors on T cells. In vitro and in vivo blockade experiments indicated that B7-H4 (also known as B7S1 or B7x) inhibits proliferation, cytokine production, and cytotoxicity of T cells. B7-H4 binds to an unknown receptor(s) that is expressed on activated T cells. However, whether B7-H4 plays nonredundant immune regulatory roles in vivo has not been tested. We generated B7-H4-deficient mice to investigate the roles of B7-H4 during various immune reactions. Consistent with its inhibitory function in vitro, B7-H4-deficient mice mounted mildly augmented T-helper 1 (Th1) responses and displayed slightly lowered parasite burdens upon Leishmania major infection compared to the wild-type mice. However, the lack of B7-H4 did not affect hypersensitive inflammatory responses in the airway or skin that are induced by either Th1 or Th2 cells. Likewise, B7-H4-deficient mice developed normal cytotoxic T-lymphocyte reactions against viral infection. Thus, B7-H4 plays a negative regulatory role in vivo but the impact of B7-H4 deficiency is minimal. These results suggest that B7-H4 is one of multiple negative cosignaling molecules that collectively provide a fine-tuning mechanism for T-cell-mediated immune responses.
Activation of T cells relies on two kinds of signals, a T-cell receptor (TCR)-mediated signal and costimulatory signals (5, 10). Costimulation is provided by costimulatory ligands that are expressed on antigen-presenting cells (APCs) and their cognate costimulatory receptors expressed on T cells. Environmental cues such as pathogens or tissue injury can stimulate APCs to upregulate a cohort of costimulatory ligands that belong to one of three groups, the B7 family, the tumor necrosis factor family, and the SLAM family (10, 27, 40, 46). Costimulation or “signal 2” was originally proposed as a positive signal that allows lymphocytes to discriminate nonself from self antigens by complementing antigen receptor signaling (signal 1) (2). However, accumulating evidence indicates that there are also negative auxiliary signals that suppress TCR-mediated signaling. Multiple layers of positive and negative “cosignaling” mechanisms appear to come into play in a coordinated manner at different times and sites of immune reactions to regulate T-cell expansion, differentiation, and survival (10, 17, 43).
Members of the B7 family can provide both positive and negative cosignaling. B7.1 and B7.2 augment TCR-mediated T-cell activation by engaging CD28, but they can also inhibit T-cell activation when they bind to cytotoxic T-lymphocyte (CTL) antigen 4, the major negative cosignaling receptor that is inducibly expressed in activated T cells (27). The inducible costimulator ligand provides a positive signal through ICOS (6, 11, 19, 20, 36). The inducible costimulator ligand-inducible costimulator signal works in synergy with B7:CD28, and it becomes critical when the B7:CD28 pathway is absent (35). We and others have shown that B7-H3 and its unknown receptor(s) downregulate T-cell responses both in vitro and in vivo (16, 23, 34). However, opposite observations have been made by other groups (3, 18, 42). It remains possible that B7-H3 binds two different receptors with opposite outcomes, depending upon the settings of immune responses. Programmed death 1 ligand 1 (PD-L1; also known as B7-H1) and PD-L2 (also known as B7-DC) play negative immune regulatory roles through binding to their shared receptor, programmed death 1 (8, 15, 21, 22). However, PD-L1 and PD-L2 may also have an unknown counterreceptor(s) providing positive cosignals (7, 28, 33, 39). Another negative cosignaling receptor, B- and T-lymphocyte attenuator (BTLA), is preferentially expressed in Th1 cells (44). Through binding to its ligand herpesvirus entry mediator, BTLA provides another layer of negative signal to T cells (26, 44). Recently, B7-H4 (also known as B7S1 or B7x) was identified as a B7 family member (24, 29, 48). In vitro T-cell assays and in vivo blockade experiments have indicated that B7-H4 inhibits CD4 and CD8 T-cell proliferation, cell cycle progression, cytokine production, and generation of CTLs. It had been suggested that BTLA may be the receptor for B7-H4 since Th1 cells from wild-type (WT) but not BTLA-deficient mice bound recombinant B7-H4 proteins (44, 48). However, a subsequent study demonstrated that BTLA does not directly bind to B7-H4 but may influence the appearance of an unknown receptor(s) for B7-H4 on the Th1 cell surface (26).
To investigate the role of B7-H4 during immune responses, we generated and characterized B7-H4-deficient mice. B7-H4 knockout (KO) mice did not show any signs of autoimmunity or disruption of immune cell homeostasis. B7-H4 KO mice mounted normal immune responses against viral infections and multiple inflammatory stimuli. A negative regulatory role of B7-H4 was found when mice were infected with Leishmania major. We propose that B7-H4 provides another layer of negative cosignaling mechanism that works in concert with a cohort of other mechanisms to fine-tune the progression of immune responses.
MATERIALS AND METHODS
Generation of B7-H4 KO mice.
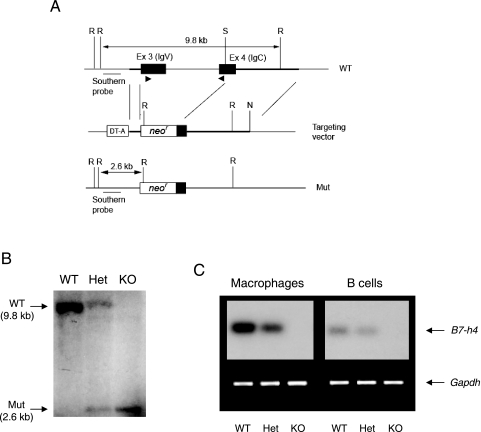
We generated an ∼11-kb contig of the mouse genomic DNA sequence comprising B7-H4 exon 3 and exon 4 by querying the mouse genomic database at the National Center for Biotechnology Information. To generate a B7-h4 targeting vector, we amplified two DNA segments from genomic DNA isolated from E14K embryonic stem (ES) cells (129/Ola) with the Expand High Fidelity PCR System (Roche), a 750-bp segment upstream of exon 3 for the short homology arm and a 3.3-kb segment containing part of exon 4 (starting from the SalI site) and its downstream sequence for the long homology arm (Fig. 1A). The PCR products were cloned into the pCR2.1 Topo-TA cloning vector (Invitrogen), and 6 (for the short arm sequence) or 10 (for the long arm sequence) clones were sequenced to obtain consensus sequences. Segments matching the consensus sequences were used for vector construction. We electroporated E14K ES cells with NotI-linearized targeting vector DNA and then selected the cells in G418 to obtain ES cell clones. We screened ES cell clones that had undergone homologous recombination by PCR and Southern blot analysis. We used three ES cell clones to generate B7-H4 KO mice by a standard protocol (34), and two of them transmitted the targeted B7-h4 allele to the germ line. We used B7-H4 KO mice and their WT littermates in a C57BL/6 × 129/Ola mixed genetic background unless indicated otherwise. All live-animal experiments were performed by protocols approved by the local Animal Care Committees (University Health Network, Toronto, Ontario, Canada; Saga University, Saga, Japan; McMaster University, Hamilton, Ontario, Canada). For Southern blot analysis, we PCR amplified an ∼1-kb DNA segment representing part of B7-h4 intron 2 from genomic DNA isolated from E14K ES cells. The PCR conditions were as follows: sense primer, 5′-CGG GGA CAT CGA GCC AGA ACT-3′; antisense primer, 5′-ACC TCC GCA TAT TCT TCA CAT-3′; 35 cycles of 94 to 60 to 72°C, 1 min each.
FIG. 1.
Generation of B7-H4 KO mice. (A) Strategy for B7-h4 gene targeting. We designed a targeting vector to replace all of B7-h4 exon (Ex) 3 (encoding the IgV domain) and one-third of exon 4 (encoding the IgC domain) with a neomycin resistance gene cassette. We prepared two homology arms by PCR as described in Materials and Methods. The location of the Southern blot probe is shown by an underline, and those of RT-PCR primers are shown by arrowheads. R, EcoRI; S, SalI; N, NotI; DT-A, diphtheria toxin A subunit gene cassette; neor, neomycin resistance gene cassette; Mut, mutant. (B) Southern blot analysis. Genomic DNAs from WT, heterozygous (Het), and KO mouse tails were digested with EcoRI and analyzed by Southern blot analysis with the probe depicted in panel A. (C) Lack of B7-H4 mRNA in cells derived from B7-H4 KO mice. First-strand cDNAs were prepared from the total RNA extracted from peritoneal macrophages and splenic B cells of WT, B7-H4 heterozygous (Het), and B7-H4 KO (KO) mice. B7-H4 cDNA was amplified by PCR along with glyceraldehyde-3-phosphate dehydrogenase cDNA as a positive control as described in Materials and Methods.
Reverse transcription (RT)-PCR.
Total RNA was isolated from CD4 T cells using Trizol reagent (Life Sciences) and subsequently reverse transcribed with a cDNA Synthesis Kit (Roche). B7-H4 cDNA was amplified by PCR (30 cycles of 94 to 60 to 72°C, 1 min each) with the glyceraldehyde-3-phosphate dehydrogenase gene as a control. We used the following primers: B7-H4 sense, 5′-GAC ATC AAA CTC AAC GGC ATC-3′; B7-H4 antisense, 5′-GCC TCG CAG CGT AAA CTC T-3′; GAPDH sense, 5′-AAT GTA TCC GTT GTG GAT CT-3′; GAPDH antisense, 5′-TCC ACC ACC CTG TTG CTG TA-3′. T-bet cDNA was amplified by PCR (35 cycles of 94 to 60 to 72°C, 1 min each) with the β-actin gene as a control. The primer sequences were as follows: T-bet sense, 5′-CTC GGA ACT CCG CTT CAT AAC TGT-3′; T-bet antisense, 5′-AAC CGC TTA TAT GTC CAC CCA GAC-3′; β-actin sense, 5′-TGG AAT CCT GTG GCA TCC ATG AAA C-3′; β-actin antisense, 5′-TAA AAC GCA GCT CAG TAA CAG TCC G-3′. B7-H4 RT-PCR products were visualized by autoradiography after hybridization of 32P-labeled B7-H4 probes.
L. major experiments.
L. major (MHOM/SU/73-5-ASKH) cells were maintained as previously described (47). Mice with a BALB/c background were subcutaneously inoculated in the right hind footpad with 5 × 106 stationary-phase promastigotes, and footpad thickness was measured every other day with a caliper. Footpad swelling was calculated by subtracting the thickness of the uninfected foot from that of the infected foot. The parasite burden was measured at day 14 postinfection as previously described (47). For cytokine analysis, single-cell suspensions of popliteal lymph node (LN) cells were made at day 14 postinfection. The LN cells (5 × 105/well) were stimulated with or without L. major antigens in the presence of irradiated (30 Gy) BALB/c splenocytes (5 × 105/well) for 3 days, and the concentrations of interleukin-4 (IL-4) and gamma interferon (IFN-γ) in the culture supernatants were measured by enzyme-linked immunosorbent assay (ELISA; R&D Systems). For analysis of T-bet expression, CD4 T cells were purified from the popliteal LNs of infected mice with magnetic beads (Miltenyi Biotech).
Airway inflammation.
We induced airway inflammation as described previously (31, 32, 34). Mice were intranasally infected with replication-defective recombinant adenoviruses carrying cDNA for granulocyte-macrophage colony-stimulating factor (GM-CSF) or a combination of GM-CSF and IL-12 at day −1. These regimens modulate the airway microenvironments such that T cells polarize toward Th2 (GM-CSF alone) or Th1 (GM-CSF plus IL-12). The mice were exposed daily for 20 min to aerosolized ovalbumin (1% [wt/vol] in 0.9% saline) for 10 consecutive days (day 0 to day 9). The extent of airway inflammation was assessed 2 days after the final exposure (day 11). The infiltrated immune cells in the airway were collected by bronchoalveolar lavage (BAL), differentially stained, and counted under a microscope. Mononuclear cells in the lung were isolated and analyzed by flow cytometry after staining with CD3, CD4 or CD8, and CD69. We measured the ex vivo concentrations of IFN-γ, IL-5, and IL-13 in BAL fluid with the Luminex 100 Total System (Luminex, Austin, TX). The splenocytes were restimulated in vitro with ovalbumin (400 μg/ml) for 5 days, and the concentrations of IFN-γ, IL-4, IL-5, IL-10, and IL-13 in the culture supernatant were measured with the Luminex 100 Total System.
Contact hypersensitivity.
To induce contact hypersensitivity reactions, we sensitized mice by applying 1% oxazolone (4-ethoxymethylene-2-phenyl-2-oxazolin-5-one; Sigma) dissolved in a mixture of acetone and olive oil (4:1) to the shaved abdomen. Five days later, the sensitized mice were challenged with the same oxazolone reagent on the right ear. Ear thickness was measured before (day 0) and after challenge exposure every 24 h with a caliper.
LCMV experiments.
For footpad swelling experiments, we injected lymphocytic choriomeningitis virus (LCMV; Armstrong strain; 3,000 PFU) into the hind footpad. Footpad thickness was measured before (day 0) and after LCMV injection (day 4 until day 15) daily with a caliper. For primary CTL responses, mice were infected with LCMV (Armstrong strain; 2,000 PFU) intravenously. Eight days later, cytotoxic activities in the spleen were measured ex vivo with 51Cr-labeled EL4 cells loaded with LCMV glycoprotein peptide p33 (KAVYNFATM) as the target (34). For secondary CTL responses, splenocytes were isolated from mice that had been infected with LCMV 30 days before. The splenocytes were restimulated in culture for 5 days in the presence of 1 μM p33 peptide and used for cytotoxicity assays as described above.
Influenza virus experiments.
CTL responses against influenza virus were monitored as previously described (1). Mice were intraperitoneally injected with influenza virus A HKx31 (x31, H3N2) (200 hemagglutinin units/mouse). In experiments assessing secondary CTL responses, we injected the mice that had been injected with x31 3 weeks before with the serologically distinct strain of influenza virus A/PR8/34 (PR8, H1N1; 200 hemagglutinin units/mouse, intraperitoneally), which shares the NP gene with x31. This method allowed us to analyze NP-specific secondary CTL responses without interference arising from the neutralizing antibody raised against x31. The expansion of NP-specific CD8 T cells in the spleen was examined by flow cytometric analysis 7 days after primary or secondary infection with Db/NP366-374 tetramers (National Institute of Allergy and Infectious Disease MHC Tetramer Core Facility) in conjunction with CD8 and CD62L. For secondary CTL responses, we measured splenic ex vivo cytotoxic activities by standard 51Cr-release assays with 51Cr-labeled EL4 cells loaded with NP366-374 peptide as the target.
Statistical analyses.
Student's t test was used to determine the statistical significance of differences between data sets.
RESULTS
Generation of B7-H4 KO mice.
B7-H4 protein is predicted to possess two extracellular immunoglobulin (Ig) domains, one transmembrane segment, and a single-amino-acid cytoplasmic tail (4, 24, 29, 48). We designed a gene-targeting strategy such that the targeted B7-h4 locus does not contain any functional exons encoding the extracellular domains of B7-H4. The two extracellular Ig domains are encoded by exon 3 and exon 4, respectively (4). We replaced all of exon 3 and the 5′ one-third of exon 4 with a neomycin resistance cassette (Fig. 1A). We obtained B7-h4-targeted mice derived from two independent ES cell clones. Both strains of mice were viable, inherited the targeted allele according to the Mendelian ratio, and grew without any developmental defects. Disruption of the B7-h4 locus was confirmed by Southern blot analysis (Fig. 1B). B7-H4 mRNA was detected by RT-PCR in WT macrophages and B cells, consistent with previous results (24). The amount of B7-H4 mRNA was reduced in a gene dosage-dependent manner in the heterozygous cells, and there was no mRNA in KO cells (Fig. 1C).
B7-H4 KO mice do not develop spontaneous autoimmune syndrome.
Disruption of key negative cosignaling receptors leads to various degrees of lymphoproliferative and/or autoimmune symptoms (21, 22, 45). Since B7-H4 has been shown to play negative regulatory roles in T-cell proliferation and function, we tested if B7-H4 KO mice develop any signs of altered immune cell differentiation and homeostasis. First, we analyzed the numbers and proportions of T, B, NK, and NKT cells and monocytes in the bone marrow, thymus, LNs, and spleen by cell counting and flow-cytometric analyses. We did not find any differences in the total cellularity or composition of immune cells in the primary and secondary lymphoid organs of WT versus B7-H4 KO mice. Furthermore, the development of thymocytes in the thymus and B-cell subsets in the bone marrow and the spleen was normal in the absence of B7-H4 (data not depicted). Second, we examined the proportion of activated T cells in the periphery by measuring the percentage of CD44hi CD62Llo T cells over the total CD4+ cells by flow cytometry. No significant difference was found between WT and B7-H4 KO mice (WT, 15.4% ± 2.9%; B7-H4 KO, 12.4% ± 2.9%; five mice of each genotype at ∼4 months of age). Third, we measured the concentrations of IgM, IgG1, IgG2a, IgG2b, IgG3, and IgA in the sera of unmanipulated WT and B7-H4 KO mice. The basal serum Ig levels of WT and B7-H4 KO mice were indistinguishable. To determine whether B7-H4 deficiency causes autoimmune symptoms, we performed two sets of experiments. First, we measured anti-single-stranded DNA autoantibodies in the serum by ELISA. Second, we histologically examined the liver, heart, kidneys, lungs, salivary glands, small intestines, large intestines, and brain to assess the level of immune cell infiltration. In both cases, we did not detect any evidence of elevated autoimmune symptoms in B7-H4 KO mice. Collectively, we conclude that lack of B7-H4 does not cause any obvious perturbations in immune cell development, homeostasis, and self-tolerance.
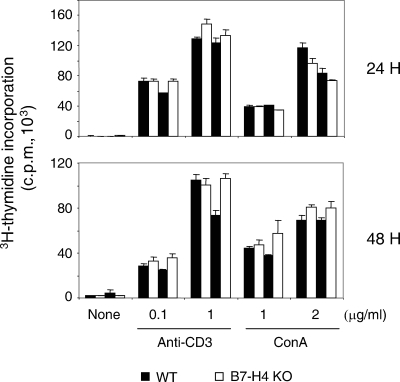
Normal T-cell proliferation in the absence of B7-H4.
It was shown that stimulation of T cells with plate-bound B7-H4Ig inhibits anti-CD3-mediated T-cell proliferation and cell cycle progression (24, 29, 48). Similarly, B7-H4 expressed on the surface of surrogate APCs inhibited T-cell proliferation (29, 48). However, it was not clear whether B7-H4 plays a nonredundant role when T cells are stimulated by APCs expressing a battery of positive and negative cosignaling ligands. To address this issue, we stimulated single-cell suspensions of the total LN cells with soluble anti-CD3 monoclonal antibody or concanavalin A. As shown in Fig. 2, we did not detect any significant increase in T-cell proliferation in cells derived from B7-H4-deficent mice compared to those from WT littermates. Thus, it appears that B7-H4 does not play a dominant regulatory role in T-cell proliferation when other cosignals are provided.
FIG. 2.
Normal T-cell proliferation in the absence of B7-H4. Single-cell suspensions of total LN cells were stimulated with soluble anti-CD3 (145-2C11; 0.1 or 1 μg/ml; BD Pharmingen) or concanavalin A (ConA; 1 or 2 μg/ml; Sigma) in U-bottom 96-well plates (1 × 105 cells/well) in triplicate. The cells were pulsed with [3H]thymidine (1 μCi/well) for the last 8 h of a 24- or 48-h incubation period. The data shown are the mean ± the standard deviation from two WT and two B7-H4 KO mice and are representative of four independent experiments.
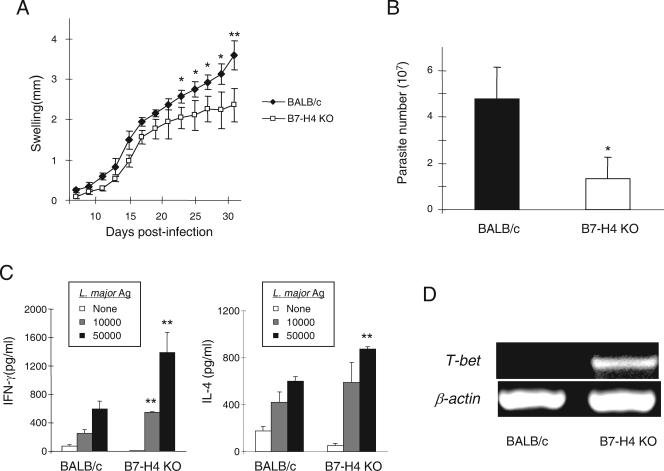
Augmented Th1 responses against L. major in B7-H4 KO mice.
Since the unknown receptor(s) for B7-H4 is expressed at a high level in Th1 cells (26, 44, 48), we reasoned that the impact of B7-H4 deficiency would be most pronounced under Th1-polarizing conditions. Therefore, we decided to test for the ability of B7-H4 KO mice to cope with L. major, control of which depends on highly polarized Th1 responses. Upon L. major infection, C57BL/6 mice control the disease with a potent Th1 response whereas BALB/c mice succumb to the disease because of a poor Th1 response. Thus, we performed our experiments with WT BALB/c and B7-H4 KO mice in a BALB/c background (backcrossed for five generations). Compared with WT BALB/c mice, B7-H4 KO mice showed an up to 30% reduction in footpad swelling, especially toward the end of disease progression (Fig. 3A). Accordingly, the number of parasites in the infected footpad was much smaller (4.76 × 107 ± 1.33 × 107 for the WT versus 1.38 × 107 ± 0.95 × 107 for the KO; n = 3; P < 0.05). These results suggest that B7-H4 might be involved in suppression of the Th1 response during L. major infection. To address this issue, we analyzed the levels of IFN-γ and IL-4 produced by T cells isolated from the draining LNs of the infected mice. Upon restimulation in vitro, T cells from B7-H4 KO mice produced an approximately twofold greater amount of IFN-γ compare to the WT counterpart (Fig. 3C, IFN-γ). In contrast, there was only marginal augmentation of IL-4 production in the same culture. The difference in IFN-γ production was reflected by the elevated level of T-bet mRNA in the CD4 T cells that were isolated from B7-H4 KO mice (Fig. 3D). We observed similar differences in L. major-induced footpad swelling between B7-h4+/− and B7-h4−/− littermates (data not depicted). Taken together, these results indicate that B7-H4 plays a negative regulatory role in Th1-mediated immune responses during L. major infection.
FIG. 3.
Enhanced Th1 responses against L. major in B7-H4 KO mice. (A) Reduced footpad swelling in B7-H4 KO mice. BALB/c or B7-H4 KO mice in a BALB/c background (n = 6) were inoculated in the right hind footpad with L. major promastigotes, and footpad swelling was measured at the indicated time points. Two mice of each genotype died at day 21 and thus were excluded from the experiment. The data shown are the mean ± the standard deviation of footpad swelling and are representative of two independent experiments. *, P < 0.05; **, P < 0.01 (Student's t test). (B) Reduced parasite burden in B7-H4 KO mice. The number of L. major promastigotes in the footpad was assessed as described in Materials and Methods at day 14 postinfection. The data shown are the mean ± the standard deviation and are representative of two independent experiments with three mice per genotype. *, P < 0.05 by Student's t test. (C) Elevated IFN-γ production by T cells from B7-H4 KO mice. Popliteal LN cells were isolated at day 14 postinfection and then restimulated with or without antigen (Ag) for 3 days. The concentrations of IFN-γ and IL-4 in the culture supernatants were measured by ELISA. The data shown are means ± standard deviations and are representative of two independent experiments with three mice per genotype. **, P < 0.01 by Student's t test compared to data from the WT control at the same dose of antigen. (D) Increased T-bet expression in CD4+ T cells of B7-H4 KO mice. CD4 T cells were purified from popliteal LNs at day 14 postinfection, and the level of T-bet mRNA was analyzed by RT-PCR as described in Materials and Methods. The data shown are representative of three experiments with three mice per genotype.
Unaltered T-cell-mediated inflammatory responses in B7-H4 KO mice.
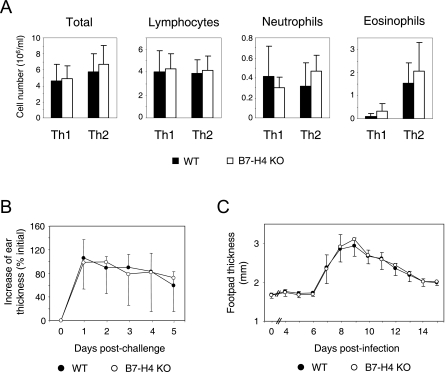
To further investigate the influence of B7-H4 deficiency on the regulation of immune responses, we used three different models of hypersensitivity reactions.
First, we used airway inflammation models in which T-helper cells can be polarized toward either Th1 or Th2 (31, 32, 34). Polarization of T-helper cells was induced by transient expression of GM-CSF and IL-12 (for Th1) or GM-CSF alone (for Th2) in the airway mucosal environment through adenoviral gene transfer. Repeated exposure to aerosolized ovalbumin leads to accumulation of immune cells in the lung. The resulting Th2-mediated airway inflammation causes prominent eosinophilia and goblet cell hyperplasia, hallmarks of human asthma (32). Under both Th1 and Th2 conditions, the numbers of total and lymphocyte, neutrophil, and eosinophil infiltrates in BAL fluid were indistinguishable between WT and B7-H4 KO mice (Fig. 4A). Ex vivo flow cytometric analysis revealed that the infiltrated T cells had similar levels of T-cell activation marker CD69 (data not depicted). Furthermore, splenocytes produced the same amounts of Th1 (IFN-γ) or Th2 cytokines (IL-4, IL-5, and IL-10) when restimulated by ovalbumin in vitro. These data indicated that the lack of B7-H4 does not affect the activation and differentiation of Th1 and Th2 cells or the overall severity of airway inflammation under our experimental conditions.
FIG. 4.
Unaltered hypersensitivity reactions in B7-H4 KO mice. (A) Airway inflammation. B7-H4 KO and WT littermate mice in a BALB/c background (backcrossed for five generations) were repeatedly exposed to aerosolized ovalbumin under Th1- or Th2-polarizing conditions as described in Materials and Methods. Immune cells in BAL fluid were enumerated after differential staining. The data shown are means ± standard deviations from a combination of two independent experiments (n = 7 WT and 10 B7-H4 KO mice for Th1 and 7 WT and 9 B7-H4 KO mice for Th2). (B) Contact hypersensitivity reaction. WT and B7-H4 KO mice were sensitized by oxazolone on the abdominal skin and then challenged on the ear skin 5 days later. The data shown represent mean percentages ± standard deviations over the initial ear thickness obtained from seven mice of each genotype. (C) LCMV-mediated footpad swelling. WT and B7-H4 KO mice were injected with LCMV in the hind footpad, and the inflammatory reaction was monitored by measuring footpad thickness. The data shown represent the mean footpad thickness of four mice of each genotype ± the standard deviation.
We also employed two T-cell-mediated delayed-type hypersensitivity models to assess the role of B7-H4 in the regulation of T-cell responses. The first model was oxazolone-mediated skin inflammation, which is known to be mediated by Th1 and CD8 T cells (41). The second model was footpad swelling in response to LCMV infection. This inflammation is known to be mediated mainly by CD8 T cells and partially by Th1 cells (9). Under both experimental conditions, B7-H4 KO mice mounted hypersensitivity reactions comparable to those of their WT control littermates (Fig. 4B and C).
Taken together, these results indicated that B7-H4 does not play any nonredundant role in the regulation of T-cell-mediated hypersensitivity reactions during multiple immunological challenges: aerosolized protein in the airway, an atopically applied chemical immunogen, and replicating virus in the footpad.
Intact antiviral CTL responses in the absence of B7-H4.
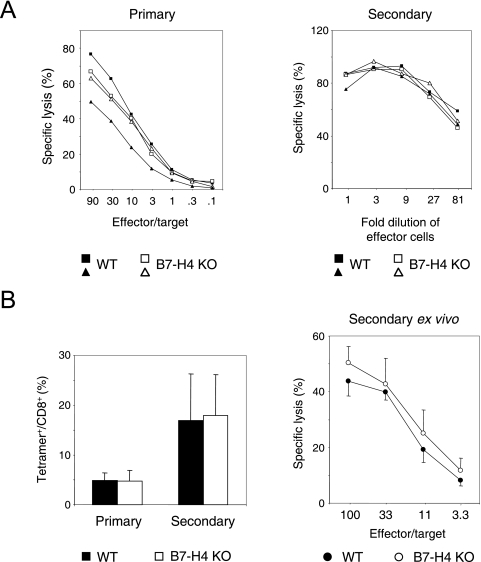
It has been shown that plate-bound B7-H4Ig or cell surface B7-H4 inhibits TCR-mediated proliferation of CD8 T cells, as well as CD4 T cells, in vitro (29, 48). In vivo blockade experiments also suggested that B7-H4 may inhibit the generation of alloreactive CTLs (29). Therefore, we tested whether B7-H4 KO mice display any alterations in CTL responses against viral infection. To this end, we employed two models of viral infection models: LCMV and influenza A virus. As shown in Fig. 5A, B7-H4 KO mice developed normal primary and secondary CTL responses, as judged by the capacity of splenocytes to lyse target cells. LCMV is known to vigorously replicate in mice and, probably by doing so, elicit a CTL response in the absence of cosignaling provided by CD28 (14). Thus, it is possible that any impact of B7-H4 deficiency could be overridden by strong TCR stimulation. In contrast to LCMV, influenza virus A HKx31 (H3N2) injected into the peritoneum of the mouse does not replicate extensively and the induction of a CTL response heavily depends on CD28-mediated cosignaling (1). Under these conditions, however, the extents of NP-specific CD8 T-cell expansion in B7-H4 KO mice upon primary and secondary influenza virus challenges were comparable to those in WT littermates, as shown by tetramer staining (Fig. 5B, left side). Consistent with the equal expansion of tetramer+ CD8 T cells, the cytotoxic activities of the splenocytes were also indistinguishable between WT and B7-H4 KO mice (Fig. 5B, right side). Collectively, these results showed that the absence B7-H4 does not affect the proliferation and differentiation of CTLs upon viral infection in vivo.
FIG. 5.
Normal antiviral CTL responses in B7-H4 KO mice. (A, left side) WT or B7-H4 KO mice (n = 2) were infected with LCMV. Splenocytes were prepared at day 8 postinfection, and ex vivo cytotoxicity was measured as described in Materials and Methods. (A, right side) WT or B7-H4 KO mice (n = 2) were infected with LCMV. Thirty days later, splenocytes were prepared and restimulated for 5 days in vitro by the addition of antigenic peptide p33. Threefold serial dilutions of the restimulated splenocytes were used as effector cells for cytotoxicity assays as described in Materials and Methods. (B, left side) Expansion of NP-specific anti-influenza virus CD8 T cells in the spleen measured 7 days after primary or secondary influenza virus infection as described in Materials and Methods. The results shown represent the average percentages of tetramer+ T cells over the total CD8 T cells in the spleen from a combination of two independent experiments (n = 10 WT and 12 B7-H4 KO mice for primary infection and 7 WT and 10 B7-H4 KO mice for secondary infection). The error bars represent standard deviations. (B, right side) Cytotoxic activities in the spleens of WT or B7-H4 KO mice 7 days after a secondary influenza virus infection measured as described in Materials and Methods. The data shown represent average specific lysis ± the standard deviation (n = four WT and seven B7-H4 KO mice).
DISCUSSION
Results of in vitro and in vivo experiments with anti-B7-H4, soluble B7-H4Ig, or cells with or without surface B7-H4 suggested that mouse B7-H4 exerts a negative influence on the proliferation of CD4 and CD8 T cells and the generation of alloreactive CTLs (24, 29, 48). To investigate the role of B7-H4 in the regulation of immune reactions in a more definitive way, we generated B7-H4 KO mice by gene targeting. Our data indicate that B7-H4 indeed plays a negative regulatory role in T-cell function. But the impact of B7-H4 deficiency is evident only in limited immunological settings.
B7-H4 KO mice develop stronger Th1 responses upon L. major infection, as evidenced by an increase in IFN-γ production and concomitant enhancement of T-bet expression by CD4 T cells. Consistent with the enhanced Th1 response, B7-H4 KO mice showed reduced footpad swelling, especially in the later phase of infection (after day 25), and a lighter parasite burden compared to BALB/c WT or littermate B7-h4+/− mice. It is possible that B7-H4 and its unknown receptor(s) are preferentially expressed in highly polarized Th1 microenvironments. This could serve as a feedback inhibitory mechanism to down-regulate the Th1 response. This view is in line with the finding that B7-H4Ig readily binds to polarized Th1 cells (26, 44, 48). Importantly, however, B7-H4 KO mice displayed normal responses to other Th1-driven immune reactions, i.e., Th1-mediated airway inflammation and oxazolone-mediated contact hypersensitivity. These findings suggest that B7-H4 is not a dominant negative regulatory factor but is rather one of the fine-tuning mechanisms (17) that cooperate with other negative regulatory molecules such as programmed death 1, BTLA, and B7-H3 (21, 22, 34, 44). The redundancy provided by other negative regulatory factors may mask the consequence of B7-H4 deficiency in multiple settings of immune reactions.
Although results from manipulation of B7-H4-mediated signals suggested that B7-H4 inhibits the proliferation of CD8 cells and the generation of CTL cytotoxicity (29, 48), our results show that lack of B7-H4 does not affect CD8 T-cell-mediated immune responses. B7-H4 KO mice developed normal CD8-mediated inflammatory footpad swelling and a CTL reaction against LCMV infection. Likewise, there were no significant alterations in either the expansion or the gain of cytotoxic effector functions of the antigen-specific CTL population during influenza virus A infection. Thus, B7-H4 does not play any nonredundant role in CD8 T-cell responses during antiviral immune reactions. It remains possible that B7-H4 plays nonredundant roles in the generation of alloreactive CTLs during graft-versus-host disease (29). This possibility will be tested with B7-H4 KO mice.
Although B7-H4 mRNA can be detected in the majority of mouse and human tissues, B7-H4 protein is expressed only in hematopoietic cells and APCs and not in normal tissues (4, 24, 29, 48). Interestingly, human B7-H4 is highly expressed in the vast majority of human breast and ovarian cancer samples and a low percentage of lung cancer tissues (4, 25, 30, 37, 38). In addition, B7-H4 expressed on a subset of tumor-associated macrophages can inhibit antitumor T-cell immunity (13). These findings suggest that B7-H4 may provide a growth advantage to tumor cells through immune evasion (12). Our B7-H4 KO mice provide an opportunity to test this hypothesis in mouse tumor models.
In sum, we demonstrated here that murine B7-H4 is another negative cosignaling molecule. B7-H4 appears to function as one of the fine-tuners of the immune system that modulate progression of immune reactions such that its nonredundant role is not readily revealed. It has yet to be tested whether B7-H4 is utilized as an immune evasion mechanism in cancer.
Acknowledgments
We thank Arda Shahinian and Jijin Shang for technical assistance.
This work was supported by grants from the Canadian Network for Vaccines and Immunotherapeutics (T.W.M.) and the Canadian Institutes of Health Research (T.H.W.). W.-K.S. was partially supported by a postdoctoral fellowship from the Cancer Research Institute. E.C. was supported by a Canada Graduate Scholarship from the Canadian Institutes of Health Research.
REFERENCES
- 1.Bertram, E. M., P. Lau, and T. H. Watts. 2002. Temporal segregation of 4-1BB versus CD28-mediated costimulation: 4-1BB ligand influences T cell numbers late in the primary response and regulates the size of the T cell memory response following influenza infection. J. Immunol. 168:3777-3785. [DOI] [PubMed] [Google Scholar]
- 2.Bretscher, P., and M. Cohn. 1970. A theory of self-nonself discrimination. Science 169:1042-1049. [DOI] [PubMed] [Google Scholar]
- 3.Chapoval, A. I., J. Ni, J. S. Lau, R. A. Wilcox, D. B. Flies, D. Liu, H. Dong, G. L. Sica, G. Zhu, K. Tamada, and L. Chen. 2001. B7-H3: a costimulatory molecule for T cell activation and IFN-γ production. Nat. Immunol. 2:269-274. [DOI] [PubMed] [Google Scholar]
- 4.Choi, I. H., G. Zhu, G. L. Sica, S. E. Strome, J. C. Cheville, J. S. Lau, Y. Zhu, D. B. Flies, K. Tamada, and L. Chen. 2003. Genomic organization and expression analysis of B7-H4, an immune inhibitory molecule of the B7 family. J. Immunol. 171:4650-4654. [DOI] [PubMed] [Google Scholar]
- 5.Collins, M., V. Ling, and B. M. Carreno. 2005. The B7 family of immune-regulatory ligands. Genome Biol. 6:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong, C., A. E. Juedes, U. A. Temann, S. Shresta, J. P. Allison, N. H. Ruddle, and R. A. Flavell. 2001. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature 409:97-101. [DOI] [PubMed] [Google Scholar]
- 7.Dong, H., G. Zhu, K. Tamada, and L. Chen. 1999. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 5:1365-1369. [DOI] [PubMed] [Google Scholar]
- 8.Freeman, G. J., A. J. Long, Y. Iwai, K. Bourque, T. Chernova, H. Nishimura, L. J. Fitz, N. Malenkovich, T. Okazaki, M. C. Byrne, H. F. Horton, L. Fouser, L. Carter, V. Ling, M. R. Bowman, B. M. Carreno, M. Collins, C. R. Wood, and T. Honjo. 2000. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 192:1027-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fung-Leung, W. P., T. M. Kundig, R. M. Zinkernagel, and T. W. Mak. 1991. Immune response against lymphocytic choriomeningitis virus infection in mice without CD8 expression. J. Exp. Med. 174:1425-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenwald, R. J., G. J. Freeman, and A. H. Sharpe. 2005. The B7 family revisited. Annu. Rev. Immunol. 23:515-548. [DOI] [PubMed] [Google Scholar]
- 11.Hutloff, A., A. M. Dittrich, K. C. Beier, B. Eljaschewitsch, R. Kraft, I. Anagnostopoulos, and R. A. Kroczek. 1999. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 397:263-266. [DOI] [PubMed] [Google Scholar]
- 12.Ichikawa, M., and L. Chen. 2005. Role of B7-H1 and B7-H4 molecules in down-regulating effector phase of T-cell immunity: novel cancer escaping mechanisms. Front. Biosci. 10:2856-2860. [DOI] [PubMed] [Google Scholar]
- 13.Kryczek, I., L. Zou, P. Rodriguez, G. Zhu, S. Wei, P. Mottram, M. Brumlik, P. Cheng, T. Curiel, L. Myers, A. Lackner, X. Alvarez, A. Ochoa, L. Chen, and W. Zou. 2006. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J. Exp. Med. 203:871-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kundig, T. M., A. Shahinian, K. Kawai, H. W. Mittrucker, E. Sebzda, M. F. Bachmann, T. W. Mak, and P. S. Ohashi. 1996. Duration of TCR stimulation determines costimulatory requirement of T cells. Immunity 5:41-52. [DOI] [PubMed] [Google Scholar]
- 15.Latchman, Y., C. R. Wood, T. Chernova, D. Chaudhary, M. Borde, I. Chernova, Y. Iwai, A. J. Long, J. A. Brown, R. Nunes, E. A. Greenfield, K. Bourque, V. A. Boussiotis, L. L. Carter, B. M. Carreno, N. Malenkovich, H. Nishimura, T. Okazaki, T. Honjo, A. H. Sharpe, and G. J. Freeman. 2001. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2:261-268. [DOI] [PubMed] [Google Scholar]
- 16.Ling, V., P. W. Wu, V. Spaulding, J. Kieleczawa, D. Luxenberg, B. M. Carreno, and M. Collins. 2003. Duplication of primate and rodent B7-H3 immunoglobulin V- and C-like domains: divergent history of functional redundancy and exon loss. Genomics 82:365-377. [DOI] [PubMed] [Google Scholar]
- 17.Loke, P., and J. P. Allison. 2004. Emerging mechanisms of immune regulation: the extended B7 family and regulatory T cells. Arthritis Res. Ther. 6:208-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo, L., A. I. Chapoval, D. B. Flies, G. Zhu, F. Hirano, S. Wang, J. S. Lau, H. Dong, K. Tamada, A. S. Flies, Y. Liu, and L. Chen. 2004. B7-H3 enhances tumor immunity in vivo by costimulating rapid clonal expansion of antigen-specific CD8+ cytolytic T cells. J. Immunol. 173:5445-5450. [DOI] [PubMed] [Google Scholar]
- 19.Mak, T. W., A. Shahinian, S. K. Yoshinaga, A. Wakeham, L. M. Boucher, M. Pintilie, G. Duncan, B. U. Gajewska, M. Gronski, U. Eriksson, B. Odermatt, A. Ho, D. Bouchard, J. S. Whorisky, M. Jordana, P. S. Ohashi, T. Pawson, F. Bladt, and A. Tafuri. 2003. Costimulation through the inducible costimulator ligand is essential for both T helper and B cell functions in T cell-dependent B cell responses. Nat. Immunol. 4:765-772. [DOI] [PubMed] [Google Scholar]
- 20.McAdam, A. J., R. J. Greenwald, M. A. Levin, T. Chernova, N. Malenkovich, V. Ling, G. J. Freeman, and A. H. Sharpe. 2001. ICOS is critical for CD40-mediated antibody class switching. Nature 409:102-105. [DOI] [PubMed] [Google Scholar]
- 21.Nishimura, H., M. Nose, H. Hiai, N. Minato, and T. Honjo. 1999. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 11:141-151. [DOI] [PubMed] [Google Scholar]
- 22.Nishimura, H., T. Okazaki, Y. Tanaka, K. Nakatani, M. Hara, A. Matsumori, S. Sasayama, A. Mizoguchi, H. Hiai, N. Minato, and T. Honjo. 2001. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 291:319-322. [DOI] [PubMed] [Google Scholar]
- 23.Prasad, D. V., T. Nguyen, Z. Li, Y. Yang, J. Duong, Y. Wang, and C. Dong. 2004. Murine B7-H3 is a negative regulator of T cells. J. Immunol. 173:2500-2506. [DOI] [PubMed] [Google Scholar]
- 24.Prasad, D. V., S. Richards, X. M. Mai, and C. Dong. 2003. B7S1, a novel B7 family member that negatively regulates T cell activation. Immunity 18:863-873. [DOI] [PubMed] [Google Scholar]
- 25.Salceda, S., T. Tang, M. Kmet, A. Munteanu, M. Ghosh, R. Macina, W. Liu, G. Pilkington, and J. Papkoff. 2005. The immunomodulatory protein B7-H4 is overexpressed in breast and ovarian cancers and promotes epithelial cell transformation. Exp. Cell Res. 306:128-141. [DOI] [PubMed] [Google Scholar]
- 26.Sedy, J. R., M. Gavrieli, K. G. Potter, M. A. Hurchla, R. C. Lindsley, K. Hildner, S. Scheu, K. Pfeffer, C. F. Ware, T. L. Murphy, and K. M. Murphy. 2005. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat. Immunol. 6:90-98. [DOI] [PubMed] [Google Scholar]
- 27.Sharpe, A. H., and G. J. Freeman. 2002. The B7-CD28 superfamily. Nat. Rev. Immunol. 2:116-126. [DOI] [PubMed] [Google Scholar]
- 28.Shin, T., G. Kennedy, K. Gorski, H. Tsuchiya, H. Koseki, M. Azuma, H. Yagita, L. Chen, J. Powell, D. Pardoll, and F. Housseau. 2003. Cooperative B7-1/2 (CD80/CD86) and B7-DC costimulation of CD4+ T cells independent of the PD-1 receptor. J. Exp. Med. 198:31-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sica, G. L., I. H. Choi, G. Zhu, K. Tamada, S. D. Wang, H. Tamura, A. I. Chapoval, D. B. Flies, J. Bajorath, and L. Chen. 2003. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity 18:849-861. [DOI] [PubMed] [Google Scholar]
- 30.Simon, I., S. Zhuo, L. Corral, E. P. Diamandis, M. J. Sarno, R. L. Wolfert, and N. W. Kim. 2006. B7-h4 is a novel membrane-bound protein and a candidate serum and tissue biomarker for ovarian cancer. Cancer Res. 66:1570-1575. [DOI] [PubMed] [Google Scholar]
- 31.Stampfli, M. R., N. G. Scott, R. E. Wiley, M. Cwiartka, S. A. Ritz, M. M. Hitt, Z. Xing, and M. Jordana. 1999. Regulation of allergic mucosal sensitization by interleukin-12 gene transfer to the airway. Am. J. Respir. Cell Mol. Biol. 21:317-326. [DOI] [PubMed] [Google Scholar]
- 32.Stampfli, M. R., R. E. Wiley, G. S. Neigh, B. U. Gajewska, X. F. Lei, D. P. Snider, Z. Xing, and M. Jordana. 1998. GM-CSF transgene expression in the airway allows aerosolized ovalbumin to induce allergic sensitization in mice. J. Clin. Investig. 102:1704-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subudhi, S. K., P. Zhou, L. M. Yerian, R. K. Chin, J. C. Lo, R. A. Anders, Y. Sun, L. Chen, Y. Wang, M. L. Alegre, and Y. X. Fu. 2004. Local expression of B7-H1 promotes organ-specific autoimmunity and transplant rejection. J. Clin. Investig. 113:694-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suh, W. K., B. U. Gajewska, H. Okada, M. A. Gronski, E. M. Bertram, W. Dawicki, G. S. Duncan, J. Bukczynski, S. Plyte, A. Elia, A. Wakeham, A. Itie, S. Chung, C. J. Da, S. Arya, T. Horan, P. Campbell, K. Gaida, P. S. Ohashi, T. H. Watts, S. K. Yoshinaga, M. R. Bray, M. Jordana, and T. W. Mak. 2003. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat. Immunol. 4:899-906. [DOI] [PubMed] [Google Scholar]
- 35.Suh, W. K., A. Tafuri, N. N. Berg-Brown, A. Shahinian, S. Plyte, G. S. Duncan, H. Okada, A. Wakeham, B. Odermatt, P. S. Ohashi, and T. W. Mak. 2004. The inducible costimulator plays the major costimulatory role in humoral immune responses in the absence of CD28. J. Immunol. 172:5917-5923. [DOI] [PubMed] [Google Scholar]
- 36.Tafuri, A., A. Shahinian, F. Bladt, S. K. Yoshinaga, M. Jordana, A. Wakeham, L. M. Boucher, D. Bouchard, V. S. Chan, G. Duncan, B. Odermatt, A. Ho, A. Itie, T. Horan, J. S. Whoriskey, T. Pawson, J. M. Penninger, P. S. Ohashi, and T. W. Mak. 2001. ICOS is essential for effective T-helper-cell responses. Nature 409:105-109. [DOI] [PubMed] [Google Scholar]
- 37.Tringler, B., W. Liu, L. Corral, K. C. Torkko, T. Enomoto, S. Davidson, M. S. Lucia, D. E. Heinz, J. Papkoff, and K. R. Shroyer. 2006. B7-H4 overexpression in ovarian tumors. Gynecol. Oncol. 100:44-52. [DOI] [PubMed] [Google Scholar]
- 38.Tringler, B., S. Zhuo, G. Pilkington, K. C. Torkko, M. Singh, M. S. Lucia, D. E. Heinz, J. Papkoff, and K. R. Shroyer. 2005. B7-h4 is highly expressed in ductal and lobular breast cancer. Clin. Cancer Res. 11:1842-1848. [DOI] [PubMed] [Google Scholar]
- 39.Tseng, S. Y., M. Otsuji, K. Gorski, X. Huang, J. E. Slansky, S. I. Pai, A. Shalabi, T. Shin, D. M. Pardoll, and H. Tsuchiya. 2001. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J. Exp. Med. 193:839-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veillette, A. 2004. SLAM family receptors regulate immunity with and without SAP-related adaptors. J. Exp. Med. 199:1175-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang, B., H. Fujisawa, L. Zhuang, I. Freed, B. G. Howell, S. Shahid, G. M. Shivji, T. W. Mak, and D. N. Sauder. 2000. CD4+ Th1 and CD8+ type 1 cytotoxic T cells both play a crucial role in the full development of contact hypersensitivity. J. Immunol. 165:6783-6790. [DOI] [PubMed] [Google Scholar]
- 42.Wang, L., C. C. Fraser, K. Kikly, A. D. Wells, R. Han, A. J. Coyle, L. Chen, and W. W. Hancock. 2005. B7-H3 promotes acute and chronic allograft rejection. Eur. J. Immunol. 35:428-438. [DOI] [PubMed] [Google Scholar]
- 43.Wang, S., and L. Chen. 2004. Co-signaling molecules of the B7-CD28 family in positive and negative regulation of T lymphocyte responses. Microbes Infect. 6:759-766. [DOI] [PubMed] [Google Scholar]
- 44.Watanabe, N., M. Gavrieli, J. R. Sedy, J. Yang, F. Fallarino, S. K. Loftin, M. A. Hurchla, N. Zimmerman, J. Sim, X. Zang, T. L. Murphy, J. H. Russell, J. P. Allison, and K. M. Murphy. 2003. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat. Immunol. 4:670-679. [DOI] [PubMed] [Google Scholar]
- 45.Waterhouse, P., J. M. Penninger, E. Timms, A. Wakeham, A. Shahinian, K. P. Lee, C. B. Thompson, H. Griesser, and T. W. Mak. 1995. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 270:985-988. [DOI] [PubMed] [Google Scholar]
- 46.Watts, T. H. 2005. TNF/TNFR family members in costimulation of T cell responses. Annu. Rev. Immunol. 23:23-68. [DOI] [PubMed] [Google Scholar]
- 47.Yoshida, H., S. Hamano, G. Senaldi, T. Covey, R. Faggioni, S. Mu, M. Xia, A. C. Wakeham, H. Nishina, J. Potter, C. J. Saris, and T. W. Mak. 2001. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity 15:569-578. [DOI] [PubMed] [Google Scholar]
- 48.Zang, X., P. Loke, J. Kim, K. Murphy, R. Waitz, and J. P. Allison. 2003. B7x: a widely expressed B7 family member that inhibits T cell activation. Proc. Natl. Acad. Sci. USA 100:10388-10392. [DOI] [PMC free article] [PubMed] [Google Scholar]