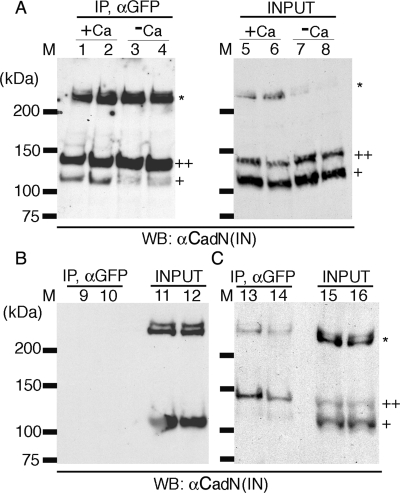
FIG. 7.
CadN mediates calcium-dependent cis interactions. (A) CadN 7b-13a-18a and CadN 7b-13a-18a-GFP (lanes 1, 3, 5, and 7) or CadN 7b-13a-18b (lanes 2, 4, 6, and 8) and its GFP-tagged version were coexpressed in S2 cells. The association of CadN and CadN-GFP, in the presence (lanes 1, 2, 5, and 6) or absence (lanes 3, 4, 7, and 8) of 5 mM of calcium, was assessed by immunoprecipitation. Extracts (lanes 5 to 8) or anti-GFP immunoprecipitates (lanes 1 to 4) were analyzed by Western blotting and probed with an antibody recognizing the CadN cytoplasmic domain [αCadN (IN)]. CadN 7b-13a-18a (or CadN 7b-13a-18b) coimmunoprecipitated with CadN 7b-13a-18a-GFP (or CadN 7b-13a-18b-GFP) in the presence of calcium. In the absence of calcium, only a small fraction of CadN coimmunoprecipitated with CadN-GFP. Mature CadN protein is proteolytically cleaved to form N-terminal and C-terminal fragments (12). The C-terminal fragments of CadN (∼110 kDa) and CadN-GFP (∼140 kDa) are indicated by single and double plus signs, respectively. Some CadN protein remained unprocessed (asterisk). (B) In the absence of CadN-GFP, CadN 7b-13a-18a (lane 9) and CadN 7b-13a-18b (lane 10) were not found in the anti-GFP immunoprecipitates, indicating that the anti-GFP antibody is specific. Extracts of the S2 cells expressing CadN 7b-13a-18a (lane 11) or CadN 7b-13a-18b (lane 12) used for the immunoprecipitate experiments were analyzed with anti-CadN (IN) Western blotting. (C) trans interactions between CadN and CadN-GFP were assessed using anti-GFP immunoprecipitation (Ip). Two separate populations of S2 cells, one expressing CadN and the other CadN-GFP (7b-13a-18a, lanes 13 and 15; 7b-13a-18b, lanes 14 and 16), were mixed and subjected to anti-GFP Ip and Western blot analysis as described before. Only a very small amount of CadN coimmunoprecipitated with CadN-GFP, indicating that the trans association between CadN and CadN-GFP was largely disrupted by the experimental procedures.