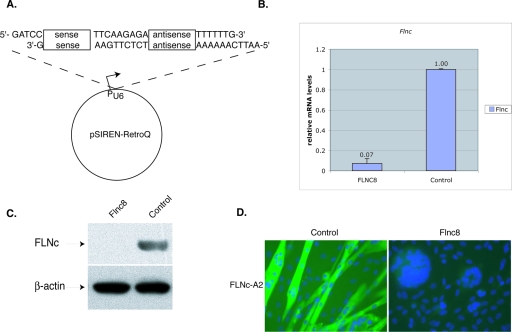
FIG. 1.
Reduction of Flnc mRNA by siRNA in the C2C12 cell line. (A) To knockdown FLNc expression, 19-bp target sequences were chosen. These sequences were used to design an inverted repeat insert and were cloned into pSirenRetroQ vector (Clontech). When the human U6 promoter is activated, this vector expresses a short hairpin RNA (shRNA). This plasmid was stably integrated into C2C12 cells using retroviral delivery. (B) The amount of Flnc knockdown was determined using quantitative RT-PCR. All of the amounts were first normalized to the endogenous Tbp (TATA binding protein) transcript levels and then to the control cell line, which expressed a shRNA that has no homology to any known mammalian sequences. The cells were allowed to differentiate for >3 days. Results shown are averages of those from five independent experiments, and the error bars represent the standard errors. The Flnc8 line shows a decrease of 93% in Flnc transcripts. (C) Ten micrograms of total protein isolated from the C2C12 cell lines was loaded onto 4 to 12% Bis-Tris gels (Invitrogen), and Western analysis was performed using FLNc-specific polyclonal antibodies. Antibodies against β-actin were used to control for even loading. The Flnc8 line has virtually no FLNc expressed. (D) Immunohistochemical analysis of FLNc expression with Flnc-A2 polyclonal antibodies (green). The Flnc8 line has virtually no FLNc protein detected, whereas in the control cell line, FLNc antibodies stain the fused fibers. Nuclei are labeled with DAPI (blue). Magnification, ×40.