Abstract
Signal transduction via guanine nucleotide binding proteins (G proteins) is involved in cardiovascular, neural, endocrine, and immune cell function. Regulators of G protein signaling (RGS proteins) speed the turn-off of G protein signals and inhibit signal transduction, but the in vivo roles of RGS proteins remain poorly defined. To overcome the redundancy of RGS functions and reveal the total contribution of RGS regulation at the Gαi2 subunit, we prepared a genomic knock-in of the RGS-insensitive G184S Gnai2 allele. The Gαi2G184S knock-in mice show a dramatic and complex phenotype affecting multiple organ systems (heart, myeloid, skeletal, and central nervous system). Both homozygotes and heterozygotes demonstrate reduced viability and decreased body weight. Other phenotypes include shortened long bones, a markedly enlarged spleen, elevated neutrophil counts, an enlarged heart, and behavioral hyperactivity. Heterozygous Gαi2+/G184S mice show some but not all of these abnormalities. Thus, loss of RGS actions at Gαi2 produces a dramatic and pleiotropic phenotype which is more evident than the phenotype seen for individual RGS protein knockouts.
Cell-cell communication is fundamental to the maintenance of homeostasis. The G protein-coupled receptor superfamily is arguably the most abundant and diverse protein family in cellular signaling and is tightly regulated. A novel family of >20 proteins termed regulators of G protein signaling, or RGS proteins, both tonically inhibit G protein function and also serve as signal control points (2, 22, 34, 39, 69). RGS-mediated inhibition of G protein signaling occurs through direct binding of the RGS protein to the Gα subunit, with subsequent GTPase-accelerating protein (GAP) actions to rapidly deactivate Gα (2). Deactivation may be accelerated up to 1,000-fold and shuts down both Gα and Gβγ signals (42, 48). RGS proteins may also competitively inhibit Gα binding to effectors such as phospholipase C (32). Most of the currently known RGS proteins interact with either Gi or Gq family G proteins and influence cyclic AMP (cAMP), Ca2+, mitogen-activated protein kinase, and ion channel signaling. There is strong evidence implicating them in the subsecond kinetics of Gi- and Go-mediated ion channel activation and deactivation in the heart (10, 21, 36) and neurons (36). In addition, the conserved RGS domain has been found to serve as a multifunctional protein adapter which can recruit many effectors or regulators to the vicinity of activated G proteins (31, 53, 62). Notable examples include p115rhoGEF (30, 40) and GRK2 (44). There is also emerging interest in RGS proteins as drug targets (9, 20, 53, 72).
However, the physiological functions of RGS proteins remain poorly defined. A number of RGS knockouts have been reported (for example, RGS1, -2, -4, and -9). The RGS9-1 knockout shows prolonged visual potentials (7), and RGS9-2 disruption results in markedly enhanced responses to drugs of abuse, such as cocaine, amphetamines, and opiates (56, 71). A human disorder, bradyopsia, with reduced visual acuity for rapidly moving objects, has been identified with loss of RGS9-1 or its membrane anchor (54). The RGS2 knockout was initially reported to have only subtle behavioral and immunologic phenotypes but was subsequently found to be markedly hypertensive (33). RGS4 (26) and RGS1 (28, 46) knockouts have subtle effects on sensorimotor functioning and lymphocyte trafficking, respectively. One difficulty in unraveling the function of RGS proteins has been the complex interactions of the many subtypes of both G proteins and RGS proteins. This redundancy of RGS function limits an understanding of the RGS role in vivo, since standard antisense or knockout strategies targeting a single RGS gene will underestimate the overall role of RGS proteins.
To determine the full contribution of RGS proteins as a group to biological responses mediated by a particular G protein (e.g., Go and Gi), we took advantage of a G alpha-subunit point mutation first found in the yeast Saccharomyces cerevisiae (19) that prevents RGS binding to the Gα subunit and GAP activity. The analogous mutation (Gly184 to Ser) in mammalian Gαo and Gαi1 prevented the GAP activity of RGS4 and RGS7 and blocked RGS4 binding to aluminum fluoride-activated Gα subunits (41). These mutations do not affect other functions of the Gα subunit, such as the intrinsic GTPase activity of the G protein or its coupling to βγ subunits, receptors, GRK, or effectors (adenylyl cyclase [AC]) (18, 25). Thus, the only known effect of the G184S mutation in Gαo and Gαi is to prevent RGS action on Gα. Several publications using overexpression of these RGS-insensitive Gα subunits revealed profound slowing of channel response kinetics and/or increased potency of agonist responses in neurons (8, 11-13, 37).
Signaling by the Gi family of “inhibitory” G proteins is complex. While they inhibit adenylyl cyclase, Gi proteins can also activate or inhibit many other effectors through either the GTP-bound Gαi or the βγ subunit released upon activation. Indeed, βγ is commonly implicated in Gi signaling and so, unlike Gαs and Gαq, overexpression of mutant Gαi proteins will not fully mimic the response. Specifically, Gβγ inhibits N-type Ca2+ channels and activates G protein-coupled inwardly rectifying K+ (GIRK) channels, phospholipase C β2, and phosphatidyl inositol 3-kinase γ isoforms (17, 51). In addition, βγ released from Gi can activate ERK through complex and cell-type-specific mechanisms (55). Along with Gq, the Gi family of G proteins is most strongly regulated by RGS proteins, and expression of Gαi2 is ubiquitous. Surprisingly, knockouts are viable (58), perhaps due to redundancy with related Gα subunits (Gαi1 and Gαi3).
In the present study, we prepared a genomic knock-in of the RGS-insensitive Gαi2G184S allele to assess the role of RGS proteins in the function of Gαi2 and to probe the physiological functions of Gαi2. A knock-in was used instead of a transgenic model to maintain the normal distribution and level of Gαi2 protein expression. Since the mutant Gαi2G184S protein can't be turned off by RGS proteins, we expected that in any tissue with functional RGS activity at Gαi2, we would see enhanced, but receptor-dependent, Gi2 activity. Indeed, the Gαi2G184S/G184S homozygous mutant mice showed enhanced signaling and a dramatic and pleiotropic physiological phenotype, including reduced viability, low birth weight, growth retardation, cardiac hypertrophy, enlarged spleen, elevated neutrophil and monocyte counts, and behavioral hyperactivity, while heterozygotes showed some elements of the phenotype.
MATERIALS AND METHODS
Materials.
Pertussis toxin (PTX) was from List Biological Laboratories (Campbell, CA), forskolin was from Calbiochem (LaJolla, CA), and isobutyl-1-methylxanthine, ATP, cAMP, and lysophosphatidic acid (LPA) were from Sigma (St. Louis, MO).
Generation of Gαi2G184S/G184S mice.
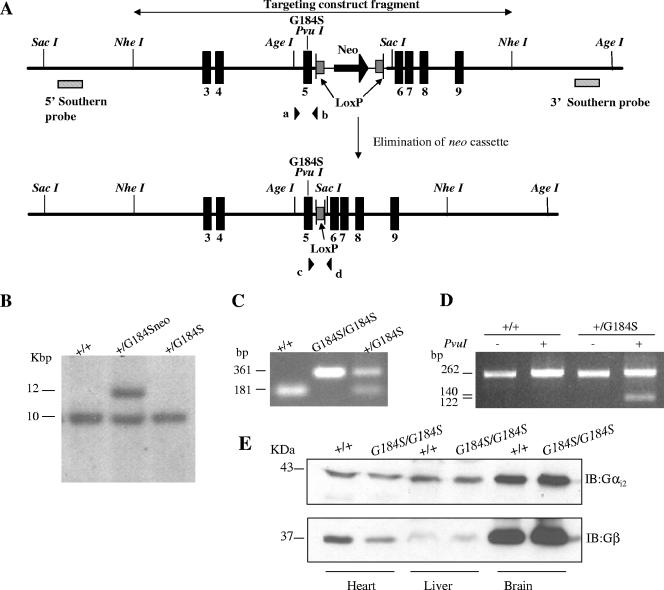
All protocols and procedures were approved by the University Committee on Use and Care of Animals, and animal care was overseen by the Unit for Laboratory Animal Medicine (University of Michigan). The production of the Gαi2G184S/G184S mice has been described in detail in the supporting online material of Fu et al. (24). Briefly, the targeting construct pTKLNL/Gαi2G184S harbors a 5′ sequence encoding thymidine kinase followed by a 13.8-kb Gαi2 genomic sequence that is divided into two regions of Gαi2 homology (6.7 and 7.1 kb in size) surrounding a loxP-flanked neo cassette. Homologous recombination between the targeting construct and the targeted locus leads to a modified Gαi2 gene (Fig. 1A) that contains the positively selectable gene neo and the G184S missense mutation located in exon 5, encoded by a GGC→TCG change, which also adds a diagnostic PvuI restriction site. Targeted CJ7 embryonic stem (ES) cells derived from 129S1/SvImJ (64) were microinjected into C57BL/6NCrl × (C57BL/6J × DBA/2J)F1 mouse blastocysts to generate ES cell-mouse chimeras. Mutant mice (Gαi2+/G184Sneo) were generated by crossing male chimeras with C57BL/6J female mice. Germ line transmission of the G184Sneo allele was identified by Southern blot detection of a 12-kb SacI band (Fig. 1B, middle lane) due to insertion of the neo cassette, while the wild-type (wt) allele shows a 10-kb band (Fig. 1B, left lane). Female heterozygous mice (Gαi2+/G184Sneo) were crossed with C57BL/6J-TgN(Zp3-cre)93Knw mice (Jax; stock no. 003651) (43) to delete the neo cassette. After one more cross with C57BL/6J mice, the offspring had lost the neo cassette, as shown by Southern blotting (Fig. 1B, right lane) and by PCR with primers c and d flanking the remaining loxP site (Fig. 1A): c, 5′-CAC ACT TCA CCT TCA AGG AC-3′; d, 5′-CTG ATG CCT AGG TGA CAG AC-3′. The wild-type allele (no loxP) produces a band of 181 bp while the inserted loxP leads to a band at 361 bp (Fig. 1C), and the intact loxP-neo-loxP allele is not detected due to the large size of the product. To ensure that the G184S mutation was carried along with the neo or loxP markers, mouse tail genomic DNA was screened by PCR amplification followed by PvuI digestion of the product. The forward primer a was 5′-GGAGCGCATTGCACAGAG-3′, and the reverse primer b was 5′-GCAGCTATGGCCCTTAAC-3′ (Fig. 1A). The appearance of the 140-bp product after PvuI digestion indicates the presence of the G184S mutation (Fig. 1D).
FIG. 1.
Gene targeting strategy and biochemical and genetic confirmation. (A) Targeted Gαi2G184Sneo genomic fragment. Top: Mouse Gαi2G184Sneo genomic fragment after homologous recombination. Homologous recombination incorporates the G-to-S mutation into the genomic DNA along with a diagnostic PvuI restriction site and a neo resistance gene flanked by loxP sites inserted in intron 5, 116 bp from the point mutation. Bottom: Scheme for removal of the neo cassette. Female heterozygous mice (Gαi2+/G184Sneo) were mated with male C57BL/6J-TgN(Zp3-cre)93Knw mice. After two generations, the neo cassette is eliminated. (B) Homologous recombination and neo removal demonstrated by Southern blot analysis in ES cells. In SacI digests, a 5′ Southern blotting probe recognizes two bands for the targeted heterozygous (+/G184Sneo) cells (middle lane) by the appearance of a 12-kb G184Sneo band, whereas only a 10-kb band is present in wild-type (+/+) cells (left lane) and cells transfected with a cre recombinase plasmid (right lane). Similar results were seen in the offspring (+/G184S) that had lost the neo cassette. (C) PCR screening confirms the in vivo loss of the neo cassette with primers (c and d) flanking the two loxP sites. For both heterozygous and homozygous mouse tail genomic DNA, a 361-bp band was generated due to the single residual LoxP site and the short linker remaining in the Gαi2 gene after Cre-recombinase action. The G184Sneo allele is not detected due to the large size of the PCR product. (D) Confirmation of the G184S mutation in mouse tail DNA. A PCR fragment amplified with primers (a and b) flanking the G184S mutation was subjected to PvuI digestion and sequencing to confirm the presence of the mutation. (E) Western blotting of Gαi2 from protein extracts from heart, liver, and brain of a wild-type and a homozygous Gαi2G184S/G184S mouse. The membrane was stripped and reblotted with a Gβ antibody as a loading control. This blot is representative of three different protein preparations.
Animals.
Heterozygous (Gαi2+/G184S) mice used in this study had been backcrossed for 4 to 7 generations onto the C57BL/6J strain. The homozygous (Gαi2G184S/G184S) mice were generated by heterozygote crosses. Animals were maintained on a 12-h light/12-h dark schedule and fed standard laboratory chow and water ad libitum. Age- and gender-matched littermates were used for all experiments. All mice used in this study were between 13 and 22 weeks of age, and the number of mice for each study is indicated in the figure legends.
Tissue and blood analyses.
Mice were sacrificed by CO2 inhalation in the morning at 20 to 22 weeks of age to obtain either blood or tissue samples. Blood was collected from the orbital sinus into EDTA dipotassium salt-coated microtubes (Sarstedt Aktlengesellschaft & Co., Germany). Tissues were removed, weighed, frozen in liquid nitrogen, and stored at −70°C until processed for analysis. The femur and tibia were dissected, and the length and width were measured using calipers. A complete blood count with differential count was performed by the Animal Diagnostic Laboratory Unit for Laboratory Animal Medicine of the University of Michigan.
G protein expression.
Tissue was taken rapidly from animals killed by CO2 inhalation. Heart, liver, and brain tissues were snap-frozen using liquid nitrogen and stored at −80°C. Extracts of tissues from control or Gαi2G184S/G184S mice were prepared as described by Koch (38), and an equal amount of protein (40 to 100 μg) from paired controls was fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted with a Gαi2-specific antiserum (J883) (49, 50) kindly provided by Susanne Mumby (University of Texas Southwestern Medical Center). The blots were then stripped and reprobed with an anti-G-protein β-subunit antibody (sc-378; Santa Cruz Biotechnology) as a control for protein loading.
MEF culture.
Mouse embryonic fibroblast (MEF) lines were isolated by trypsinization of separate embryos at embryonic day 13.5 as described elsewhere (66). Homogenous cell suspensions were maintained and expanded at 37°C in high-glucose Dulbecco's modified Eagle's medium (DMEM; Gibco BRL) supplemented with 10% (vol/vol) bovine serum (Gibco BRL). A 3T3 protocol (66) was performed to establish cell lines; that is, every 3 days, cells were trypsinized and counted, and 3 × 105 cells were plated per 60-mm dish. Three each of the Gαi2 wild-type, heterozygous (+/G184S), and homozygous (G184S/G184S) cell lines were established.
Whole-cell cAMP production.
cAMP production in MEF cells (see “MEF culture,” above) was determined in 24-well plates as described by Wade et al. (68), and inhibition by LPA was assessed. Briefly, MEF cells (passage 5 and below) were plated at 20,000 cells per well and incubated with 1 μCi/well [3H]adenine for 18 to 20 h. Cells were washed once with DMEM, and cAMP accumulation was initiated by adding DMEM containing 1 mM isobutyl-1-methylxanthine and 10 μM forskolin with the indicated concentrations of LPA. After 30 min at 37°C, acid-soluble nucleotides were collected and separated on Dowex and alumina columns as described previously (60).
ERK and Akt phosphorylation.
MEF cells (passage 30 or above; see “MEF culture,” above) were seeded in six-well culture plates and incubated at 37°C in a humidified incubator containing 5% CO2 in air until ∼80% confluent. Cells were then incubated with DMEM supplemented with 0.5% fetal bovine serum overnight to minimize basal Akt and ERK phosphorylation. For PTX treatment, 100 ng/ml PTX was included during the overnight serum starvation. After activation with the indicated concentrations of LPA for 7.5 min, medium was removed, and cells were washed with cold phosphate-buffered saline before addition of phospho-homogenization buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM Na2EDTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, and 1 μg/ml leupeptin). Homogenates were scraped into 1.5-ml tubes and centrifuged (13,793 × g, 20 min, 4°C), and supernatants were retained for phosphoprotein analysis. Samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 12% gels and transferred to Immobilon-P, probed with monoclonal antibodies against phospho-ERK1/2 (Thr202/Tyr204; Cell Signaling Technology, Beverly, MA) and phospho-Akt (Ser473; Cell Signaling Technology), and visualized with chemiluminescence. Bands were quantitated on a Kodak Image Station 440CF and analyzed with the Kodak 1D Image Analysis software. The blot was subsequently stripped and reprobed with ERK- and Akt-specific antibodies (Cell Signaling Technology) to assess total kinase expression. Ratios of phosphorylated versus total kinase are averages of three separate samples which were run and blotted under identical conditions.
Telemetric measurement of heart rate and activity.
A biocompatible ETA-F20 radiotransmitter (Data Sciences International, St. Paul, MN) was implanted intraabdominally into male mice (about 20 g body weight) under isoflurane anesthesia. After surgery, mice were allowed to recover for 14 days. Physical activity index, electrocardiographic traces, and body temperature were recorded for 5 min every hour for 72 h via telemetry in individually housed, nonanesthetized, freely moving animals. Data were acquired and analyzed using Dataquest A.R.T. 3.1 software (Data Science International).
Echocardiography.
Mice were sedated with 1.5% isoflurane and placed in a supine position. Two-dimensionally guided M-mode recordings were obtained by an experienced operator as previously described (4). Scans were from a short-axis view at the level of the papillary muscles with either an Acuson Sequoia system with an Acuson 15-MHz linear-array transducer or a GE Vivid 7 system with a GE S10-MHz phased-array transducer (General Electric).
Statistical analyses.
Comparisons of individual group means used a two-tailed Student's t test. Two-way analysis of variance (ANOVA) with Bonferroni posttest was used to compare multiple data sets. All statistical calculations were done using GraphPad Prism version 4 (GraphPad Software Inc., San Diego, Calif.).
RESULTS AND DISCUSSION
Gαi2G184S knock-in mice are viable but at low rates.
To elucidate the in vivo role of RGS proteins in the function of Gαi2, genomic knock-in mice carrying the RGS-insensitive mutant Gαi2G184S allele were generated. The mutant mice show early lethality, as the genotypes do not appear in the expected Mendelian ratios (Table 1). Both homozygous and heterozygous Gαi2G184S mice obtained from heterozygote crosses are underrepresented at weaning (3 weeks of age) (Table 1). There are only one-third as many homozygotes (G184S/G184S) as wild type (+/+), and even the heterozygotes (+/G184S) are found at a frequency lower than expected (1.33 times the +/+ frequency, rather than twice their number). The deviation from the expected ratio is highly significant (χ2 of 47.5, df 2; P < 0.0001). The reduced proportion of +/G184S mice from +/G184S × +/G184S matings (1.33 times the number of +/+ littermates instead of 2 times; χ2 of 13.6, df 1; P < 0.0002) probably involves both fetal and maternal contributions, as the +/+ × +/G184S matings show only a small and not statistically significant reduction in heterozygote offspring (0.86 of wt). Furthermore, there is some neonatal lethality, as we have seen evidence of fetal resorption (both resorbed fetuses were G184S/G184S genotype) and reduced numbers of homozygotes in embryonic day 13.5 MEFs (10:16:4 for +/+, +/G184S, and G184S/G184S). While the low number of homozygous embryos is not statistically significant due to the small n, the proportions (1.6× +/+ for +/G184S and 0.4× +/+ for G184S/G184S) are quite similar to those seen at weaning. The presence of a phenotype in heterozygotes as well as in homozygotes is expected given the gain-of-function nature of the G protein mutation. It is also consistent with our previously reported findings in cardiocytes differentiated from ES cells, where the Gαo+/G184S heterozygotes showed strongly enhanced negative chronotropic responses to the A1 adenosine agonist PIA (25). The phenotype of mutant mice is not due to changes in the level of expression, since Gαi2 protein amounts are comparable in heart, liver, and brain of wild-type and homozygous Gαi2G184S/G184S mice (Fig. 1E).
TABLE 1.
Genotype frequencies of Gαi2+/G184S × Gαi2+/G184S matingsa
| Genotype | Gαi2+/G184S × Gαi2+/G184S
|
Gαi2+/G184S × C57BL/6J
|
||
|---|---|---|---|---|
| nb | % with genotypec (ratio to +/+) | nb | % with genotypec (ratio to +/+) | |
| +/+ | 145 | 37.5 (1.00) | 37 | 53.9 (1.00) |
| +/G184S | 193 | 49.9 (1.33) | 33 | 46.1 (0.86) |
| G184S/G184S | 49 | 12.7 (0.34) | ||
The number (and ratio to +/+) of animals with the indicated genotype at weaning (3 weeks) is shown. Gαi2+/G184S mice for the Gαi2+/G184S × Gαi2+/G184S cross are from the N4 generation against C57BL/6J. Gαi2+/G184S mice from the Gαi2+/G184S × C57BL/6J cross are N5 against C57BL/6J.
Number of animals in each genotype includes both males and females; there was no difference in sex ratio distribution.
The observed proportions of the three genotypes from Gαi2+/G184S × Gαi2+/G184S matings are highly significantly different from the expected 1:2:1 proportions (χ2, 47.5; P < 0.0001). The ratio of +/+ to heterozygotes (+/G184S) from the +/G184S × +/G184S crosses (1:1.33) is also significantly different (χ2, 13.6; P < 0.0002). However, heterozygotes from the Gαi2+/G184S × C57BL/6J cross are slightly reduced but not significantly.
Enhanced signaling through the Gi-coupled LPA receptor in Gαi2G184S mouse embryonic fibroblasts.
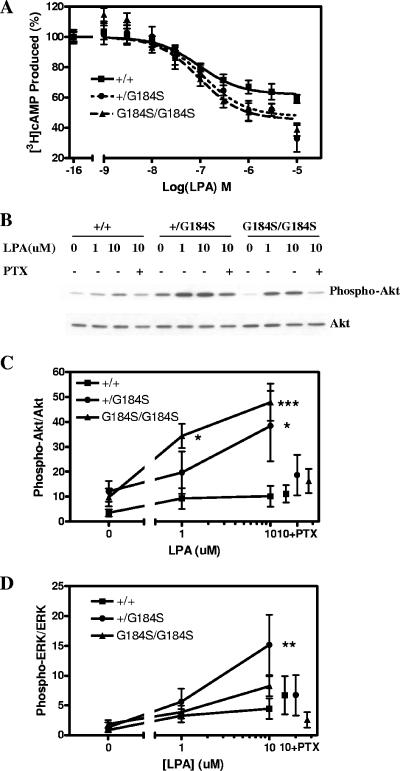
To assess the biochemical function of the mutant Gαi2, MEFs derived from wild-type, heterozygous, and homozygous littermate embryos were prepared. LPA inhibits AC and acts as a strong mitogen towards fibroblasts and other cell types via pertussis toxin-sensitive processes (63). LPA-mediated inhibition of AC was measured in embryonic fibroblasts derived from both the homozygous and heterozygous mice (Fig. 2A). Forskolin-stimulated AC was unchanged (data not shown), but LPA-mediated inhibition was enhanced. Maximum inhibition of forskolin-stimulated AC by LPA was 37% for wild-type MEFs and 52% and 54% for Gαi2+/G184S and Gαi2G184S/G184S, respectively (n = 6 to 9; P < 0.05, t test). In contrast to results for adenosine and carbachol signaling in ES-derived cardiocytes with this mutation (24), there was no significant shift in the 50% effective concentration for LPA-mediated AC inhibition (71, 95, and 80 nM for +/+, +/G184S, and G184S/G184S, respectively), and so the only change was in the maximum response.
FIG. 2.
Biochemical phenotype of Gαi2G184S mouse embryonic fibroblast cells. (A) Lysophosphatidic acid inhibition of forskolin-stimulated AC. Dose-response curves for inhibition of AC by lysophosphatidic acid were determined from three individual embryonic fibroblast cell lines of each genotype. n = 9 for both +/+ and +/G184S, and n = 6 for G184S/G184S. Maximum inhibition among all three genotypes is different by F-test, P < 0.005. (B) PTX-sensitive phosphorylation/activation of Akt stimulated by LPA. MEF cells were stimulated with the indicated concentrations of LPA for 7.5 min. Cell lysates were analyzed by immunoblotting with an Akt phospho-specific antibody (Ser473). The membrane was stripped and reprobed with an antibody raised against total Akt. In some samples, the cells were incubated with PTX (100 ng/ml) overnight before stimulation with 10 μM LPA. (C and D) Western blotting and densitometric analysis of activation of Akt (C) and ERK (D) from averages of three independent experiments. The ratios of the densities of phosphorylated Akt or ERK to those for total Akt or ERK were calculated and are means ± standard errors of the means of three experiments. Significant differences from +/+ cells by two-way ANOVA with Bonferroni posttest are indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
We also examined Akt and ERK activation in response to LPA. There is a modest LPA-mediated stimulation of Akt phosphorylation in wild-type MEFs, but this is markedly increased in both Gαi2+/G184S and Gαi2G184S/G184S MEFs (Fig. 2B and C). In contrast, ERK activation by LPA was only slightly enhanced by the Gαi2G184S allele, if at all (Fig. 2D). Interestingly, previous studies failed to show LPA-induced activation of Akt in Rat-1 embryo fibroblasts (6) and showed only a modest enhancement in NIH 3T3 cells (23), and so the dramatic effect in our mutant cell lines is striking. For Akt, there was also a small increase in basal phosphorylation (when assessed as a ratio of P-Akt to Akt), but this was not statistically significant. It may be due to residual LPA present even at low (0.5%) serum concentrations. Importantly, the enhancement of Akt activation by LPA in the mutant cell lines was blocked by PTX pretreatment, while PTX did not significantly affect stimulation in the wt cells. Thus, Akt activation by LPA receptors and Gi2 is strongly regulated by RGS proteins, and in the absence of RGS effects on Gαi2 there is a shift from a non-PTX-sensitive process to a predominantly Gi-mediated Akt activation in the mutant cell lines.
The effects of Gαi2 with the G184S mutation differ from those of the constitutively active GTPase-deficient Gαi2Q205L allele in two respects. First, our Gαi2G184S is still under control of receptors, and so it provides a hyperactive but more physiological stimulus pattern. Second, βγ signaling is also enhanced with Gαi2G184S, since loss of RGS-mediated GAP activity leads to a prolonged Gα-GTP state with a concomitant increase in free or active βγ, while expression of the Gα12Q205L mutant should not increase the amount of free βγ but only produces stimuli mediated by the Gα subunit. Consistent with this, we found that receptor-dependent Gi regulation of both adenylyl cyclase and Akt in MEFs was significantly enhanced by either one or two copies of this Gαi2G184S mutation, with the βγ-regulated phosphatidylinositol 3-kinase/Akt pathway being more strongly enhanced.
Growth retardation in Gαi2G184S knock-in mice.
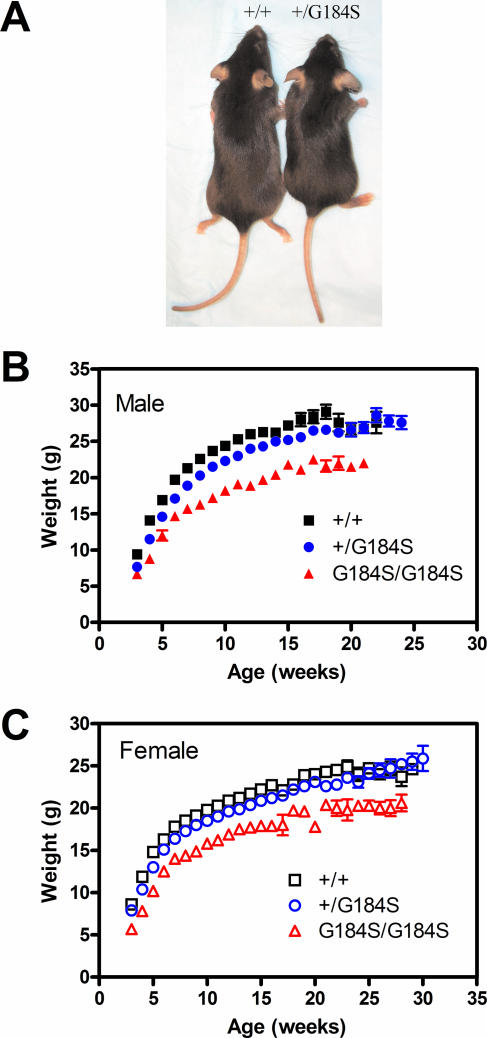
The Gαi2G184S knock-in mice showed a much more dramatic and complex phenotype affecting multiple organ systems (heart, myeloid, skeletal, and central nervous system) compared to the limited phenotype of Gαi2-deficient mice (Table 2). The most apparent physiological phenotype of Gαi2G184S/G184S mice is their small size (Fig. 3A). At 3 weeks of age, Gαi2G184S/G184S mice had significantly reduced body weights (females [P < 0.001] and males [P < 0.05] by two-way ANOVA with Bonferroni posttest) compared to age- and sex-matched +/+ controls (Fig. 3B and C). The heterozygotes also showed significantly reduced body weights from 4 to 14 weeks for both males and females (P < 0.001 to 0.05). With aging, the body weight of Gαi2G184S/G184S mice partially catches up with that of +/+ controls, but a significant difference still remains at 18 to 20 weeks (P < 0.01). Interestingly, mice deficient for Gαi2 also displayed growth retardation that was apparent at 6 weeks of age (59), indicating that Gαi2 plays an important role in both embryogenesis and neonatal growth, as described previously (47, 65). The pattern of low birth weight with significant “catch-up” for the Gαi2G184S/G184S mice is different from dwarf mice with a growth hormone receptor knockout (GHR−/−), where weights are nearly normal at birth but rapidly lose ground to their wild-type littermates (15). The pattern seen for the Gαi2G184S/G184S mice is more reminiscent of the transgenic growth hormone antagonist-expressing mice (15). Reduced growth hormone could contribute to the small size, but the increased activity could also contribute (see below). There was not, however, any significant difference in either food intake or O2 consumption (not shown).
TABLE 2.
Comparison of phenotypes of RGS-insensitive Gαi2G184S mice and Gαi2-deficient mice
| Mouse type | Group | Phenotype |
|---|---|---|
| RGS-insensitive Gαi2G184S | General | Pre-/neonatal lethality (homozygote Gαi2G184S/G184S and heterozygote Gαi2+/G184S) |
| Low body weight | ||
| Short bones | ||
| Hematologic | Large spleen | |
| Increased monocyte and neutrophil counts | ||
| Cardiac | Daytime tachycardia | |
| Increased heart organ weight/body weight ratio | ||
| Hyperdynamic function | ||
| Neurobehavioral | Hyperactivity | |
| Gαi2 deficient | Generala | Pre- and postnatal lethality |
| Growth retardation apparent at 6 wks of age | ||
| Pathologica | Ulcerative colitis and adenocarcinoma of colon | |
| Immunologica,b | Increased no. of single positive (mature) T cells with high-intensity CD3 staining in thymus but unaffected lymphocyte homing in spleen | |
| Impaired marginal zone and B-1 B-cell development | ||
| Hematologicc | Impaired platelet activation | |
| Cardiacd | Attenuated muscarinic inhibition of contractility and calcium currents in adult cardiomyocytes |
FIG. 3.
Gαi2G184S mice are small. (A) Five-week-old homozygous (right) and wt (left) male mice are shown. (B and C) wt (+/+), heterozygote (+/G184S), and homozygote (G184S/G184S) mice were weighed weekly, and means ± standard deviations are shown for male (B) and female (C) animals. Numbers of animals were as follows: +/+, 54 males, 57 females; +/G184S, 74 males, 78 females; G184S/G184S, 18 males, 21 females. Both homozygous and heterozygous mice had significantly reduced body weights compared to age- and sex-matched wt controls (see text for details).
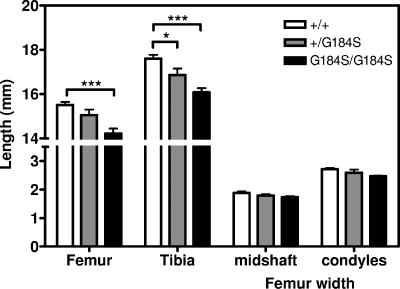
Consistent with their decreased body weight, Gαi2G184S/G184S mice also showed reduced body length (91.8 ± 1.1 [n = 10] versus 98.6 ± 0.7 for +/+ [n = 9]; P < 0.001, t test) and shorter bones (Fig. 4). They have a significant reduction in femur and tibia length compared with those of age- and sex-matched +/+ controls (Fig. 4). However, the size and morphology of the tibial growth plates were not significantly altered at 12 weeks of age (data not shown). One possible mechanism of the bone abnormalities could be a pseudohypoparathyroidism-like reduction in cAMP production. Patients with PHP-1a, which is caused by a heterozygous loss of function of Gαs, have skeletal abnormalities, reduced responsiveness to parathyroid hormone, reduced serum calcium levels, and often mental retardation (1). A mouse model with a heterozygous mutant Gnas1 shows similar effects (70). Our Gαi2G184S/G184S mice do not appear to be parathyroid hormone resistant, as they have normal serum calcium levels (not shown). Other features of PHP, such as obesity, subcutaneous ossifications, and brachydactyly, were also not observed.
FIG. 4.
Gαi2G184S mice have skeletal abnormalities. Male mice of three genotypes (+/+, n = 5; +/G184S, n = 2; G184S/G184S, n = 5) were sacrificed, and bone length and width were measured using calipers. The genotype effect was highly significant by two-way ANOVA (P < 0.0001), and the tibia length was significantly shorter for mutant mice by a Bonferroni posttest (*, P < 0.05; ***, P < 0.001).
Hematologic abnormalities in Gαi2G184S knock-in mice.
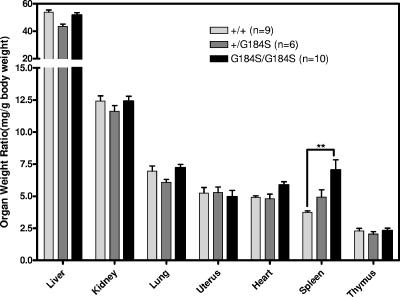
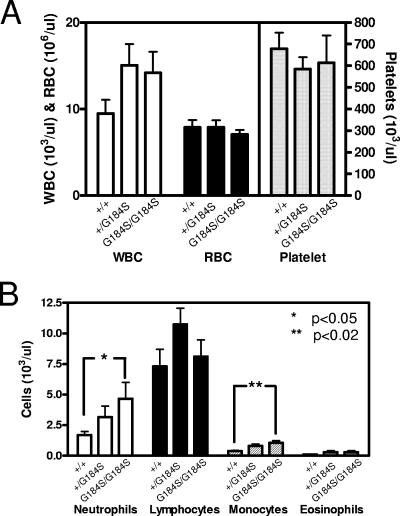
Another striking observation was the marked splenomegaly in homozygous mutant mice (P < 0.01 by two-way ANOVA with Bonferroni posttest) (Fig. 5). The spleen/body weight ratio for female Gαi2G184S/G184S mice (7.0 ± 0.80 mg/g) at 20 to 22 weeks of age was nearly double that of +/+ controls (3.7 ± 0.12 mg/g). A similar increase was also observed in males (not shown). In contrast, most other tissues (such as liver, kidney, lung, and uterus) retained their normal proportion to body weight. Peripheral blood counts showed no significant differences in total white blood cell, red blood cell, and platelet counts (Fig. 6A). However, homozygous Gαi2G184S/G184S mice showed significantly elevated absolute neutrophil and monocyte counts (Fig. 6B) compared with wild-type mice. This result suggests an enhanced myeloid lineage differentiation, which would not be surprising given the role of Gαi2 in neutrophil and monocyte function (3). Furthermore, transgenic expression of SDF-1/CXCL12 was recently shown to prevent myeloid precursor apoptosis in vitro and to increase myeloid differentiation in vivo (5) by a mechanism involving CXCR4 and Gαi. Since some endogenous SDF1a is found in myeloid precursor cells (27), the potentiated Gαi2 signaling in our Gαi2G184S/G184S mutant mice could have an effect similar to that which occurs with increased SDF-1/CXCL12 expression in the transgenic mice. Thus, increased survival and myelopoeisis may underlie the increased spleen size and peripheral neutrophil and monocyte counts seen in the mutant mice.
FIG. 5.
Normalized organ weights as percentages of body weight. Female wild-type (+/+), heterozygote (+/G184S), and homozygote G184S/G184S mice were sacrificed between 20 and 22 weeks, organs were weighed, and weight ratios were calculated as mg organ weight/g body weight. **, P < 0.01 by two-way ANOVA with Bonferroni posttest.
FIG. 6.
Gαi2G184S/G184S mice have elevated neutrophil and monocyte counts. Blood drawn from male and female mice (+/+, n = 13; +/G184S, n = 4; G184S/G184S, n = 13) via the lateral saphenous vein or from the orbital sinus of animals at sacrifice was collected in heparinized capillary tubes (Becton Dickson), and complete blood counts were done on a Coulter apparatus. Smears were stained, and differential counts were performed manually. Data are means ± standard errors of the means, and statistical significance of differences between +/+ and G184S/G184S was determined by t test (*, P < 0.05; **, P < 0.02).
Homozygous Gαi2G184S mice are hyperactive.
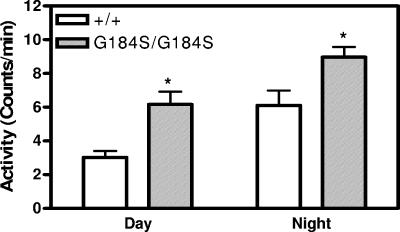
Another significant effect is neurobehavioral. Based on data obtained during telemetry electrocardiographic monitoring, we observed that the Gαi2G184S/G184S mice were more active during both day and night (Fig. 7). This is intriguing, since a knockout of RGS9 (which affects primarily Gαo and is localized to certain dopamine-related brain regions) shows an increased activity response to amphetamines and cocaine but no difference in unstimulated physical activity detected by beam-breaking in an activity chamber (56).
FIG. 7.
Homozygous Gαi2G184S/G184S mice are hyperactive. Activity data were captured for eight consecutive days with the activity detection feature of electrocardiography frequency transmitter (DSI) monitors on awake and unrestrained mice 16 weeks of age. Data are means ± standard errors of the means. Two-way ANOVA shows a significant effect of both genotype and time of day (P < 0.0005). *, P < 0.05 by Bonferroni posttest. n = 5.
Cardiovascular alterations in Gαi2G184S knock-in mice.
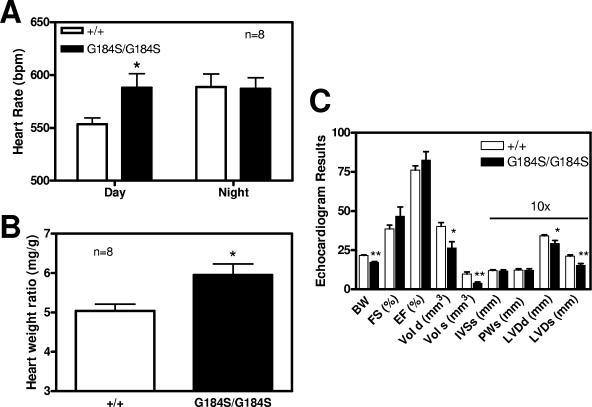
In addition to the spleen, the heart was the one other organ that appeared to show an increase in mass relative to body weight in the mutants. This was true in females, with a 19% increase in heart weight/body weight ratio (P < 0.01 by t test) (Fig. 5), and was confirmed in males, with an 18% increase (P < 0.02 by t test) (Fig. 8B). In contrast to the spleen, there was not an increase in heart size in heterozygotes, though this could be due to the small magnitude of the effect of the mutation on heart size, even in homozygotes.
FIG. 8.
Cardiovascular studies with homozygous Gαi2G184S/G184S mice. (A) Gαi2G184S/G184S mice have an increased daytime heart rate. Implanted electrocardiography frequency transmitters recorded the heart rate for 48 h, and the average heart rate was calculated for day and night from eight animals of each genotype. The G184S/G184S mutants showed increased daytime heart rate (P < 0.05). The larger error value for G184S/G184S in the daytime was due to one individual with a very high heart rate, but the difference was still significant (P < 0.05) with that value dropped. (B) Gαi2G184S/G184S mice have enlarged hearts. Male mice (eight G184S/G184S and eight +/+ littermate controls) showed an increased heart weight/body weight ratio, confirming the observation in females (Fig. 5). The effect in males (20%) is of approximately the same magnitude as in females (19% increase; P < 0.02 by t test in Fig. 5). The difference between +/+ and G184S/G184S males is statistically significant (P < 0.02 by t test). (C) Echocardiographic measurements. Seven female +/+ and six female G184S/G184S mice at 13 to 15 weeks were studied under isoflurane anesthesia. Values of cardiac volume and left ventricular (LV) diameter, intraventricular septum and posterior wall were measured and fractional shortening and ejection fraction calculated. BW, body weight; FS, fractional shortening; EF, ejection fraction; Vol d, LV diastolic volume; Vol s, LV systolic volume; IVSs, intraventricular septum in systole; PWs, posterior wall in systole; LVDd, LV dimension in diastole; LVDs, LV dimension in systole. Values are means ± standard errors of the means, with statistical comparisons done by t test. *, P < 0.05; **, P < 0.01. n = 7.
Along with the increased heart weight, the Gαi2G184S/G184S homozygous mice showed an increase in baseline heart rate during the daytime (Fig. 8A). They also showed a hyperdynamic echocardiographic profile with a marked decrease in end systolic volume and a smaller but also statistically significant decrease in end diastolic volume (Fig. 8C). While fractional shortening and ejection fraction were slightly but not significantly increased, the end systolic volume even when corrected for body weight was dramatically reduced (0.23 versus 0.44 mm3/g). The increased heart rate and evidence of increased contractility on echo suggest increased sympathetic tone. Indeed, the tachycardia in Gαi2G184S/G184S mice was unexpected, since Gi mediates GIRK activation, which slows SA node rates. The increased heart rate was especially surprising in the face of our recent demonstration (24) that Gαi2G184S/G184S mutant mice have dramatically enhanced muscarinic cholinergic bradycardia, which should lead to increased parasympathetic, rather than sympathetic, tone. Furthermore, cardiac overexpression of Gαs leads to tachycardia (67), while enhanced Gαi function should have the opposite effect. Thus, the increased heart rate is likely due to central nervous system effects—also reflected in the behavioral hyperactivity—rather than effects on cardiac function per se. Also, hyperactivation of Gi in heart by overexpression of a modified kappa opioid receptor (Ro1) leads to conduction defects and dilated cardiomyopathy, which appears to be opposite from our phenotype. Two differences in the models could account for this discrepancy (57). The overexpression of Ro1 could produce effects on its own, just as the tTA itself can lead to cardiomyopathy (45). Also, our enhanced Gi function may be more modest, given the need for a physiological stimulus to produce a response.
Given the increased heart size, we thus add Gαi2 to the list of G proteins (including Gαs and Gαq) whose enhanced signaling leads to cardiac hypertrophy. It could be related to either systemic alterations, such as increased sympathetic tone, or to enhanced intrinsic cardiac signaling through ERK (29, 61) or Akt (14) activation, which can both cause cardiac hypertrophy. If this were due to intrinsic cardiac signaling, it would probably be mediated by the βγ subunit, which is the primary signaling molecule to ERK and Akt, since the Gαi subunit generally does not activate those pathways. We did find a significantly enhanced PTX-sensitive LPA-induced Akt activation in MEFs from these mice, consistent with this possibility.
Summary.
The RGSi Gαi2G184S mice described here demonstrate a major contribution of the RGS protein family to control of signaling by Gi2 in multiple organ systems. Significant increases in AC inhibition and Akt activation were observed, while ERK signaling seemed less affected, at least in MEFs. Predicted results were seen, such as the recently reported enhancement of muscarinic bradycardia (24) and alterations in myeloid activity, along with results contrary to expectations, such as tachycardia, behavioral hyperactivity, and cardiac hypertrophy. Also, unexpected effects on bone and general growth/metabolic function were revealed. Furthermore, Gαi2 regulation by RGS proteins is important during development, as there is substantial neonatal lethality in the Gαi2G184S/G184S mice and some even in heterozygotes. These mice and the general approach of using RGS-insensitive Gα subunits, besides demonstrating the role of RGS proteins, provide novel insights into subtype-selective signaling by Gi family G proteins, where other approaches such as expression of constitutively active Gα subunits will not reveal key functions mediated by βγ release. Furthermore, RGS-insensitive Gα subunits can help dissect the function of the similar and partially redundant Gαi1, Gαi2, and Gαi3 proteins. We do not yet have a full picture of all of the physiological changes in these mice or a complete understanding of the precise mechanisms of all of the alterations, but the dramatic and pleiotropic phenotype reveals a substantial role for RGS proteins and Gαi2 in a broad range of physiological functions.
Acknowledgments
This work was supported by NIH grants R01-GM39561 (R.R.N.), Multidisciplinary Cardiovascular Research Training grant NIH T32 HL07853-06 at the University of Michigan (X.H.), and an American Heart Association Predoctoral Fellowship (Y.F.) and was also supported in part by the Michigan Diabetes, Research, and Training Center (NIH P60 DK20572), the University of Michigan Cancer Center (NIH P30 CA046592), the University of Rheumatic Diseases Center Core (NIH P30AR0483), the University of Michigan Gastrointestinal Hormone Research Core Center (NIH P30DK034933), and the Michigan Animal Models Consortium funded by the Michigan Economic Development Corporation and the Michigan Technology Tri-Corridor (grant 085P1000815).
We sincerely thank Susanne Mumby of the University of Texas Southwestern Medical Center for providing Gαi2-specific antibody, Kimber Converso for performing the cardiac echo studies, and Min Liu for assistance in this project.
REFERENCES
- 1.Bastepe, M., and H. Juppner. 2005. GNAS locus and pseudohypoparathyroidism. Horm. Res. 63:65-74. [DOI] [PubMed] [Google Scholar]
- 2.Berman, D. M., T. Kozasa, and A. G. Gilman. 1996. The GTPase-activating protein RGS4 stabilizes the transition state for nucleotide hydrolysis. J. Biol. Chem. 271:27209-27212. [DOI] [PubMed] [Google Scholar]
- 3.Bokoch, G. M., and A. G. Gilman. 1984. Inhibition of receptor-mediated release of arachidonic acid by pertussis toxin. Cell 39:301-308. [DOI] [PubMed] [Google Scholar]
- 4.Boluyt, M. O., K. Converso, H. S. Hwang, A. Mikkor, and M. W. Russell. 2004. Echocardiographic assessment of age-associated changes in systolic and diastolic function of the female F344 rat heart. J. Appl. Physiol. 96:822-828. [DOI] [PubMed] [Google Scholar]
- 5.Broxmeyer, H. E., S. Cooper, L. Kohli, G. Hangoc, Y. Lee, C. Mantel, D. W. Clapp, and C. H. Kim. 2003. Transgenic expression of stromal cell-derived factor-1/CXC chemokine ligand 12 enhances myeloid progenitor cell survival/antiapoptosis in vitro in response to growth factor withdrawal and enhances myelopoiesis in vivo. J. Immunol. 170:421-429. [DOI] [PubMed] [Google Scholar]
- 6.Burgering, B. M., and P. J. Coffer. 1995. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 376:599-602. [DOI] [PubMed] [Google Scholar]
- 7.Chen, C. K., M. E. Burns, W. He, T. G. Wensel, D. A. Baylor, and M. I. Simon. 2000. Slowed recovery of rod photoresponse in mice lacking the GTPase accelerating protein RGS9-1. Nature 403:557-560. [DOI] [PubMed] [Google Scholar]
- 8.Chen, H., and N. A. Lambert. 2000. Endogenous regulators of G protein signaling proteins regulate presynaptic inhibition at rat hippocampal synapses. Proc. Natl. Acad. Sci. USA 97:12810-12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho, H., K. Harrison, and J. H. Kehrl. 2004. Regulators of G protein signaling: potential drug targets for controlling cardiovascular and immune function. Curr. Drug Targets Immune Endocr. Metab. Disord. 4:107-118. [DOI] [PubMed] [Google Scholar]
- 10.Chuang, H. H., M. Yu, Y. N. Jan, and L. Y. Jan. 1998. Evidence that the nucleotide exchange and hydrolysis cycle of G proteins causes acute desensitization of G-protein gated inward rectifier K+ channels. Proc. Natl. Acad. Sci. USA 95:11727-11732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark, M. J., C. Harrison, H. Zhong, R. R. Neubig, and J. R. Traynor. 2003. Endogenous RGS protein action modulates mu-opioid signaling through Gαo. Effects on adenylyl cyclase, extracellular signal-regulated kinases, and intracellular calcium pathways. J. Biol. Chem. 278:9418-9425. [DOI] [PubMed] [Google Scholar]
- 12.Clark, M. J., R. R. Neubig, and J. R. Traynor. 2004. Endogenous regulator of G protein signaling proteins suppress Gαo-dependent, mu-opioid agonist-mediated adenylyl cyclase supersensitization. J. Pharmacol. Exp. Ther. 310:215-222. [DOI] [PubMed] [Google Scholar]
- 13.Clark, M. J., and J. R. Traynor. 2005. Endogenous regulator of G protein signaling proteins reduce μ-opioid receptor desensitization and down-regulation and adenylyl cyclase tolerance in C6 cells. J. Pharmacol. Exp. Ther. 312:809-815. [DOI] [PubMed] [Google Scholar]
- 14.Condorelli, G., A. Drusco, G. Stassi, A. Bellacosa, R. Roncarati, G. Iaccarino, M. A. Russo, Y. Gu, N. Dalton, C. Chung, M. V. Latronico, C. Napoli, J. Sadoshima, C. M. Croce, and J. Ross, Jr. 2002. Akt induces enhanced myocardial contractility and cell size in vivo in transgenic mice. Proc. Natl. Acad. Sci. USA 99:12333-12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coschigano, K. T., A. N. Holland, M. E. Riders, E. O. List, A. Flyvbjerg, and J. J. Kopchick. 2003. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology 144:3799-3810. [DOI] [PubMed] [Google Scholar]
- 16.Dalwadi, H., B. Wei, M. Schrage, K. Spicher, T. T. Su, L. Birnbaumer, D. J. Rawlings, and J. Braun. 2003. B cell developmental requirement for the G alpha i2 gene. J. Immunol. 170:1707-1715. [DOI] [PubMed] [Google Scholar]
- 17.Dascal, N. 2001. Ion-channel regulation by G proteins. Trends Endocrinol. Metab. 12:391-398. [DOI] [PubMed] [Google Scholar]
- 18.Day, P. W., J. J. Tesmer, R. Sterne-Marr, L. C. Freeman, J. L. Benovic, and P. B. Wedegaertner. 2004. Characterization of the GRK2 binding site of Gαq. J. Biol. Chem. 279:53643-53652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DiBello, P. R., T. R. Garrison, D. M. Apanovitch, G. Hoffman, D. J. Shuey, K. Mason, M. I. Cockett, and H. G. Dohlman. 1998. Selective uncoupling of RGS action by a single point mutation in the G protein alpha-subunit. J. Biol. Chem. 273:5780-5784. [DOI] [PubMed] [Google Scholar]
- 20.Doggrell, S. A. 2004. Is RGS-2 a new drug development target in cardiovascular disease? Expert Opin. Ther. Targets 8:355-358. [DOI] [PubMed] [Google Scholar]
- 21.Doupnik, C. A., N. Davidson, H. A. Lester, and P. Kofuji. 1997. RGS proteins reconstitute the rapid gating kinetics of Gβγ-activated inwardly rectifying K+ channels. Proc. Natl. Acad. Sci. USA 94:10461-10466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Druey, K. M., K. J. Blumer, V. H. Kang, and J. H. Kehrl. 1996. Inhibition of G-protein-mediated MAP kinase activation by a new mammalian gene family. Nature 379:742-746. [DOI] [PubMed] [Google Scholar]
- 23.Fang, X., S. Yu, R. LaPushin, Y. Lu, T. Furui, L. Z. Penn, D. Stokoe, J. R. Erickson, R. C. Bast, Jr., and G. B. Mills. 2000. Lysophosphatidic acid prevents apoptosis in fibroblasts via Gi-protein-mediated activation of mitogen-activated protein kinase. Biochem. J. 352:135-143. [PMC free article] [PubMed] [Google Scholar]
- 24.Fu, Y., X. Huang, H. Zhong, R. M. Mortensen, L. D'Alecy, and R. R. Neubig. 2006. Endogenous RGS proteins and Gα subtypes differentially control muscarinic and adenosine-mediated chronotropic effects. Circ. Res. 67:266-274. [DOI] [PubMed] [Google Scholar]
- 25.Fu, Y., H. Zhong, M. Nanamori, R. M. Mortensen, X. Huang, K. Lan, and R. R. Neubig. 2004. RGS-insensitive G-protein mutations to study the role of endogenous RGS proteins. Methods Enzymol. 389:229-243. [DOI] [PubMed] [Google Scholar]
- 26.Grillet, N., A. Pattyn, C. Contet, B. L. Kieffer, C. Goridis, and J. F. Brunet. 2005. Generation and characterization of Rgs4 mutant mice. Mol. Cell. Biol. 25:4221-4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo, Y., G. Hangoc, H. Bian, L. M. Pelus, and H. E. Broxmeyer. 2005. SDF-1/CXCL12 enhances survival and chemotaxis of murine embryonic stem cells and production of primitive and definitive hematopoietic progenitor cells. Stem Cells 23:1324-1332. [DOI] [PubMed] [Google Scholar]
- 28.Han, S. B., C. Moratz, N. N. Huang, B. Kelsall, H. Cho, C. S. Shi, O. Schwartz, and J. H. Kehrl. 2005. Rgs1 and Gnai2 regulate the entrance of B lymphocytes into lymph nodes and B cell motility within lymph node follicles. Immunity 22:343-354. [DOI] [PubMed] [Google Scholar]
- 29.Harris, I. S., S. Zhang, I. Treskov, A. Kovacs, C. Weinheimer, and A. J. Muslin. 2004. Raf-1 kinase is required for cardiac hypertrophy and cardiomyocyte survival in response to pressure overload. Circulation 110:718-723. [DOI] [PubMed] [Google Scholar]
- 30.Hart, M. J., X. Jiang, T. Kozasa, W. Roscoe, W. D. Singer, A. G. Gilman, P. C. Sternweis, and G. Bollag. 1998. Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Gα13. Science 280:2112-2114. [DOI] [PubMed] [Google Scholar]
- 31.Hepler, J. R. 2003. RGS protein and G protein interactions: a little help from their friends. Mol. Pharmacol. 64:547-549. [DOI] [PubMed] [Google Scholar]
- 32.Hepler, J. R., D. M. Berman, A. G. Gilman, and T. Kozasa. 1997. RGS4 and GAIP are GTPase-activating proteins for Gqα and block activation of phospholipase Cβ by γ-thio-GTP-Gqα. Proc. Natl. Acad. Sci. USA 94:428-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heximer, S. P., R. H. Knutsen, X. Sun, K. M. Kaltenbronn, M. H. Rhee, N. Peng, A. Oliveira-dos-Santos, J. M. Penninger, A. J. Muslin, T. H. Steinberg, J. M. Wyss, R. P. Mecham, and K. J. Blumer. 2003. Hypertension and prolonged vasoconstrictor signaling in RGS2-deficient mice. J. Clin. Investig. 111:1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hollinger, S., and J. R. Hepler. 2002. Cellular regulation of RGS proteins: modulators and integrators of G protein signaling. Pharmacol. Rev. 54:527-559. [DOI] [PubMed] [Google Scholar]
- 35.Jantzen, H. M., D. S. Milstone, L. Gousset, P. B. Conley, and R. M. Mortensen. 2001. Impaired activation of murine platelets lacking Gαi2. J. Clin. Investig. 108:477-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeong, S. W., and S. R. Ikeda. 2001. Differential regulation of G protein-gated inwardly rectifying K+ channel kinetics by distinct domains of RGS8. J. Physiol. 535:335-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeong, S. W., and S. R. Ikeda. 2000. Endogenous regulator of G-protein signaling proteins modify N-type calcium channel modulation in rat sympathetic neurons. J. Neurosci. 20:4489-4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koch, W. J., H. A. Rockman, P. Samama, R. A. Hamilton, R. A. Bond, C. A. Milano, and R. J. Lefkowitz. 1995. Cardiac function in mice overexpressing the beta-adrenergic receptor kinase or a beta ARK inhibitor. Science 268:1350-1353. [DOI] [PubMed] [Google Scholar]
- 39.Koelle, M. R., and H. R. Horvitz. 1996. EGL-10 regulates G protein signaling in the C. elegans nervous system and shares a conserved domain with many mammalian proteins. Cell 84:115-125. [DOI] [PubMed] [Google Scholar]
- 40.Kozasa, T., X. Jiang, M. J. Hart, P. M. Sternweis, W. D. Singer, A. G. Gilman, G. Bollag, and P. C. Sternweis. 1998. p115 RhoGEF, a GTPase activating protein for Gα12 and Gα13. Science 280:2109-2111. [DOI] [PubMed] [Google Scholar]
- 41.Lan, K. L., N. A. Sarvazyan, R. Taussig, R. G. Mackenzie, P. R. DiBello, H. G. Dohlman, and R. R. Neubig. 1998. A point mutation in Gαo and Gαi1 blocks interaction with regulator of G protein signaling proteins. J. Biol. Chem. 273:12794-12797. [DOI] [PubMed] [Google Scholar]
- 42.Lan, K. L., H. Zhong, M. Nanamori, and R. R. Neubig. 2000. Rapid kinetics of regulator of G-protein signaling (RGS)-mediated Gαi and Gαo deactivation. Gα specificity of RGS4 AND RGS7. J. Biol. Chem. 275:33497-33503. [DOI] [PubMed] [Google Scholar]
- 43.Lewandoski, M., K. M. Wassarman, and G. R. Martin. 1997. Zp3-cre, a transgenic mouse line for the activation or inactivation of loxP-flanked target genes specifically in the female germ line. Curr. Biol. 7:148-151. [DOI] [PubMed] [Google Scholar]
- 44.Lodowski, D. T., J. A. Pitcher, W. D. Capel, R. J. Lefkowitz, and J. J. Tesmer. 2003. Keeping G proteins at bay: a complex between G protein-coupled receptor kinase 2 and Gβγ. Science 300:1256-1262. [DOI] [PubMed] [Google Scholar]
- 45.McCloskey, D. T., L. Turnbull, P. M. Swigart, A. C. Zambon, S. Turcato, S. Joho, W. Grossman, B. R. Conklin, P. C. Simpson, and A. J. Baker. 2005. Cardiac transgenesis with the tetracycline transactivator changes myocardial function and gene expression. Physiol. Genomics 22:118-126. [DOI] [PubMed] [Google Scholar]
- 46.Moratz, C., K. Harrison, and J. H. Kehrl. 2004. Regulation of chemokine-induced lymphocyte migration by RGS proteins. Methods Enzymol. 389:15-32. [DOI] [PubMed] [Google Scholar]
- 47.Moxham, C. M., Y. Hod, and C. C. Malbon. 1993. Induction of G alpha i2-specific antisense RNA in vivo inhibits neonatal growth. Science 260:991-995. [DOI] [PubMed] [Google Scholar]
- 48.Mukhopadhyay, S., and E. M. Ross. 1999. Rapid GTP binding and hydrolysis by Gq promoted by receptor and GTPase-activating proteins. Proc. Natl. Acad. Sci. USA 96:9539-9544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mumby, S. M., and A. G. Gilman. 1991. Synthetic peptide antisera with determined specificity for G protein alpha or beta subunits. Methods Enzymol. 195:215-233. [DOI] [PubMed] [Google Scholar]
- 50.Mumby, S. M., R. A. Kahn, D. R. Manning, and A. G. Gilman. 1986. Antisera of designed specificity for subunits of guanine nucleotide-binding regulatory proteins. Proc. Natl. Acad. Sci. USA 83:265-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murga, C., L. Laguinge, R. Wetzker, A. Cuadrado, and J. S. Gutkind. 1998. Activation of Akt/protein kinase B by G protein-coupled receptors. A role for alpha and beta gamma subunits of heterotrimeric G proteins acting through phosphatidylinositol-3-OH kinase γ. J. Biol. Chem. 273:19080-19085. [DOI] [PubMed] [Google Scholar]
- 52.Nagata, K., C. Ye, M. Jain, D. S. Milstone, R. Liao, and R. M. Mortensen. 2000. Gαi2 but not Gαi3 is required for muscarinic inhibition of contractility and calcium currents in adult cardiomyocytes. Circ. Res. 87:903-909. [DOI] [PubMed] [Google Scholar]
- 53.Neubig, R. R., and D. P. Siderovski. 2002. Regulators of G-protein signalling as new central nervous system drug targets. Nat. Rev. Drug Disc. 1:187-197. [DOI] [PubMed] [Google Scholar]
- 54.Nishiguchi, K. M., M. A. Sandberg, A. C. Kooijman, K. A. Martemyanov, J. W. Pott, S. A. Hagstrom, V. Y. Arshavsky, E. L. Berson, and T. P. Dryja. 2004. Defects in RGS9 or its anchor protein R9AP in patients with slow photoreceptor deactivation. Nature 427:75-78. [DOI] [PubMed] [Google Scholar]
- 55.Pace, A. M., M. Faure, and H. R. Bourne. 1995. Gi2-mediated activation of the MAP kinase cascade. Mol. Biol. Cell 6:1685-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rahman, Z., J. Schwarz, S. J. Gold, V. Zachariou, M. N. Wein, K. H. Choi, A. Kovoor, C. K. Chen, R. J. DiLeone, S. C. Schwarz, D. E. Selley, L. J. Sim-Selley, M. Barrot, R. R. Luedtke, D. Self, R. L. Neve, H. A. Lester, M. I. Simon, and E. J. Nestler. 2003. RGS9 modulates dopamine signaling in the basal ganglia. Neuron 38:941-952. [DOI] [PubMed] [Google Scholar]
- 57.Redfern, C. H., M. Y. Degtyarev, A. T. Kwa, N. Salomonis, N. Cotte, T. Nanevicz, N. Fidelman, K. Desai, K. Vranizan, E. K. Lee, P. Coward, N. Shah, J. A. Warrington, G. I. Fishman, D. Bernstein, A. J. Baker, and B. R. Conklin. 2000. Conditional expression of a Gi-coupled receptor causes ventricular conduction delay and a lethal cardiomyopathy. Proc. Natl. Acad. Sci. USA 97:4826-4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rudolph, U., M. J. Finegold, S. S. Rich, G. R. Harriman, Y. Srinivasan, P. Brabet, G. Boulay, A. Bradley, and L. Birnbaumer. 1995. Ulcerative colitis and adenocarcinoma of the colon in G alpha i2-deficient mice. Nat. Genet. 10:143-150. [DOI] [PubMed] [Google Scholar]
- 59.Rudolph, U., M. J. Finegold, S. S. Rich, G. R. Harriman, Y. Srinivasan, P. Brabet, A. Bradley, and L. Birnbaumer. 1995. Gi2 alpha protein deficiency: a model of inflammatory bowel disease. J. Clin. Immunol. 15:101S-105S. [DOI] [PubMed] [Google Scholar]
- 60.Salomon, Y., C. Londos, and M. Rodbell. 1974. A highly sensitive adenylate cyclase assay. Anal. Biochem. 58:541-548. [DOI] [PubMed] [Google Scholar]
- 61.Sanna, B., O. F. Bueno, Y. S. Dai, B. J. Wilkins, and J. D. Molkentin. 2005. Direct and indirect interactions between calcineurin-NFAT and MEK1-extracellular signal-regulated kinase 1/2 signaling pathways regulate cardiac gene expression and cellular growth. Mol. Cell. Biol. 25:865-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Siderovski, D. P., B. Strockbine, and C. I. Behe. 1999. Whither goest the RGS proteins? Crit. Rev. Biochem. Mol. Biol. 34:215-251. [DOI] [PubMed] [Google Scholar]
- 63.Swarthout, J. T., and H. W. Walling. 2000. Lysophosphatidic acid: receptors, signaling and survival. Cell Mol. Life Sci. 57:1978-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Swiatek, P. J., and T. Gridley. 1993. Perinatal lethality and defects in hindbrain development in mice homozygous for a targeted mutation of the zinc finger gene Krox20. Genes Dev. 7:2071-2084. [DOI] [PubMed] [Google Scholar]
- 65.Takano, K., S. Asano, and N. Yamashita. 1994. Activation of G protein-coupled K+ channels by dopamine in human GH-producing cells. Am. J. Physiol. Endocrinol. Metab. 266:E318-E325. [DOI] [PubMed] [Google Scholar]
- 66.Todaro, G. J., and H. Green. 1963. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J. Cell Biol. 17:299-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Uechi, M., K. Asai, M. Osaka, A. Smith, N. Sato, T. E. Wagner, Y. Ishikawa, H. Hayakawa, D. E. Vatner, R. P. Shannon, C. J. Homcy, and S. F. Vatner. 1998. Depressed heart rate variability and arterial baroreflex in conscious transgenic mice with overexpression of cardiac Gsα. Circ. Res. 82:416-423. [DOI] [PubMed] [Google Scholar]
- 68.Wade, S. M., W. K. Lim, K. L. Lan, D. A. Chung, M. Nanamori, and R. R. Neubig. 1999. Gi activator region of α2A-adrenergic receptors: distinct basic residues mediate Gi versus Gs activation. Mol. Pharmacol. 56:1005-1013. [DOI] [PubMed] [Google Scholar]
- 69.Watson, N., M. E. Linder, K. M. Druey, J. H. Kehrl, and K. J. Blumer. 1996. RGS family members: GTPase-activating proteins for heterotrimeric G-protein alpha-subunits. Nature 383:172-175. [DOI] [PubMed] [Google Scholar]
- 70.Yu, S., D. Yu, E. Lee, M. Eckhaus, R. Lee, Z. Corria, D. Accili, H. Westphal, and L. S. Weinstein. 1998. Variable and tissue-specific hormone resistance in heterotrimeric Gs protein alpha-subunit (Gsα) knockout mice is due to tissue-specific imprinting of the Gsα gene. Proc. Natl. Acad. Sci. USA 95:8715-8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zachariou, V., D. Georgescu, N. Sanchez, Z. Rahman, R. DiLeone, O. Berton, R. L. Neve, L. J. Sim-Selley, D. E. Selley, S. J. Gold, and E. J. Nestler. 2003. Essential role for RGS9 in opiate action. Proc. Natl. Acad. Sci. USA 100:13656-13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhong, H., and R. R. Neubig. 2001. Regulator of G protein signaling proteins: novel multifunctional drug targets. J. Pharmacol. Exp. Ther. 297:837-845. [PubMed] [Google Scholar]