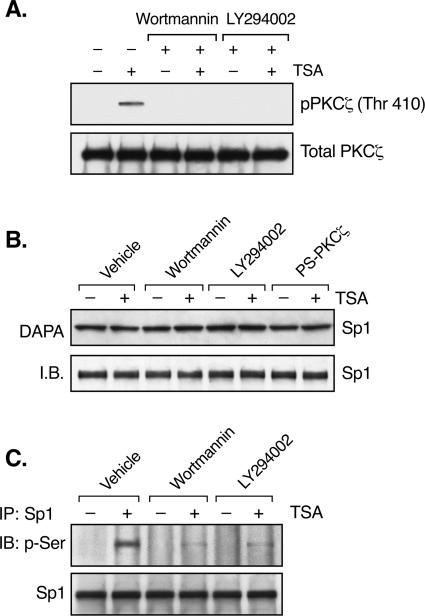
FIG. 7.
PI3K activity is required for PKCζ activation and Sp1 phosphorylation. (A) JAR cells were pretreated with or without wortmannin (0.5 μM) or LY294002 (50 μM) for 1 h, followed by incubation with or without TSA (100 ng/ml) for 24 h. Whole-cell lysates were immunoprecipitated with PKCζ antibody, followed by immunoblotting (IB) with phospho-PKCζ antibody (Thr 410). The total amounts of PKCζ precipitated from these cells are also shown as a control. (B) DAPA of Sp1 binding activity in JAR cells. Nuclear extracts isolated from JAR cells treated with or without TSA in the presence of wortmannin, LY294002, or PS-PKCζ were incubated with the biotin-labeled proximal Sp1 site of the LHR gene promoter, followed by immunoblotting with Sp1 antibody. The level of Sp1 protein is also shown. (C) IP of the nuclear extracts from panel A by Sp1 antibody was followed by immunoblotting with an antibody recognizing p-Ser and with an Sp1 antibody, respectively.