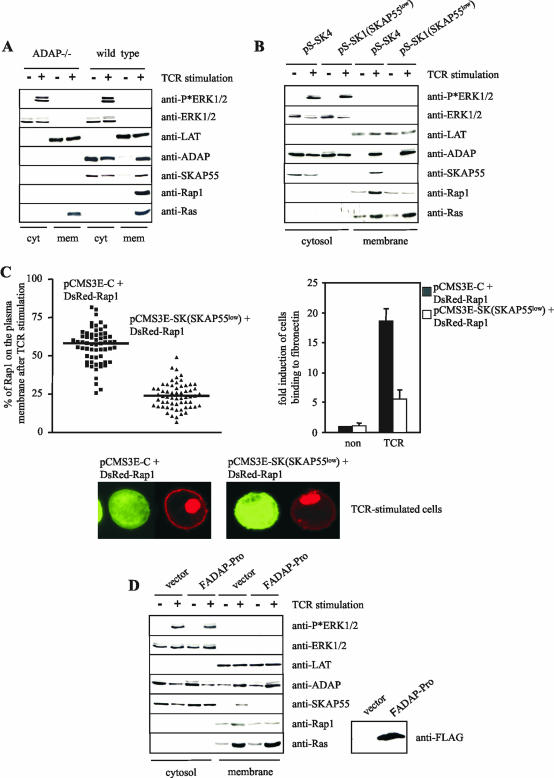
FIG.7.
Disruption of the ADAP/SKAP55 module interferes with the plasma membrane localization of Rap1. (A) Purified splenic T cells prepared from ADAP-deficient (ADAP−/−) or wild-type mice were either left unstimulated (−) or stimulated with CD3 MAb 2C11 (+) for 5 min at 37°C. Subsequently, membrane and cytosolic fractions were prepared from unstimulated (−) or stimulated (+) cells. Note that the membrane fraction was strongly enriched in plasma membranes, as indicated by the presence of Na+/K+ ATPase, the transmembrane adapter protein LAT, and almost complete absence of the endosomal marker EEA1. The individual samples were analyzed by Western blotting using anti-ADAP, anti-SKAP55, anti-Rap1, and anti-Ras antibodies. To control the fractionation efficiency and proper TCR stimulation, fractions were assessed for the presence of LAT (plasma membrane), ERK1/2 (cytosol), or phospho-ERK1/2 (stimulation). (B) Jurkat T cells were transiently transfected with either pS-SK4 (scrambled) or pS-SK1 (siRNA of SKAP55 [SKAP55low]) and were either left unstimulated (−) or were stimulated for 5 min with the CD3 MAb C305. Cytosolic and membrane fractions were analyzed as described above. (C) Jurkat T cells were transiently transfected with either pCMSE-C (scrambled) or pCMS3-SK (siRNA of SKAP55 [SKAP55low]) in combination with DsRed-tagged Rap1. Cells were stimulated for 5 min using CD3 MAb C305 and were imaged with a confocal laser-scanning microscope. Images are representative of three independent experiments. The graph indicates the percentage of Rap1 translocated on the plasma membrane of a total of 60 cells analyzed in three independent experiments. In parallel, transfectants were analyzed for their capability to adhere to fibronectin (unstimulated [non] or stimulated for 30 min with CD3 MAb OKT3 [TCR]). Adhesion data represent the means and SE of three independent experiments. (D) Jurkat T cells were transiently transfected with either empty vector or FADAP-Pro. Cells were either left unstimulated (−) or were stimulated using CD3 MAb C305 (+), and cytosolic and membrane fractions were subsequently prepared. The overexpression of FADAP-Pro was assessed by anti-FLAG Western blotting.