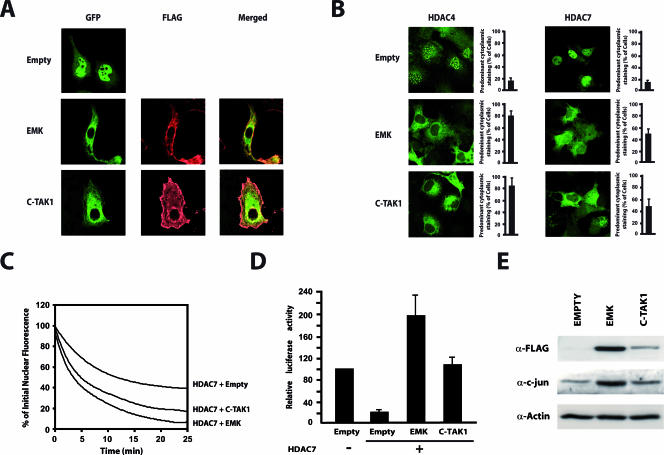
FIG. 7.
EMK and C-TAK1 control nuclear export of class IIa HDACs and regulate their repressive activity. (A) Cos7 cells were transfected with expression vectors for GFP-HDAC7 and FLAG-tagged EMK or C-TAK1. The intracellular localization of HDAC7 was detected by direct immunofluorescence (GFP) while MARKs were revealed by indirect immunofluorescence using an AlexaFluor 568-labeled anti-FLAG antibody (FLAG). (B) Cos7 cells were transfected with expression vectors for GFP-HDAC4 or GFP-HDAC7 and constructs for EMK or C-TAK1. The subcellular distribution of the GFP-fused HDACs proteins was examined by confocal immunofluorescence microscopy. Bar histograms represent the mean percentages of cells showing predominant cytoplasmic staining. (C) A construct encoding GFP-HDAC7 was transfected into Cos7 cells, either with expressing vectors for EMK, C-TAK1, or a control plasmid (Empty). The cytoplasm of GFP-positive cells was repeatedly bleached, and FLIP measurements were performed as described in Materials and Methods. Analyses of the FLIP data from three independent experiments are shown as fit curves. (D) Do11.10 cells were transiently transfected with a luciferase reporter plasmid driven by the Nur77 promoter and the expression plasmids for the indicated proteins. Luciferase activities are presented relative to the basal luciferase activity of the reporter. Results are from five independent experiments, each performed in triplicate. (E) Total cellular lysates were prepared from Cos7 cells transiently expressing FLAG-tagged versions of EMK and C-TAK1. Cell lysates were examined by Western blotting with antisera for c-jun, FLAG, and actin.