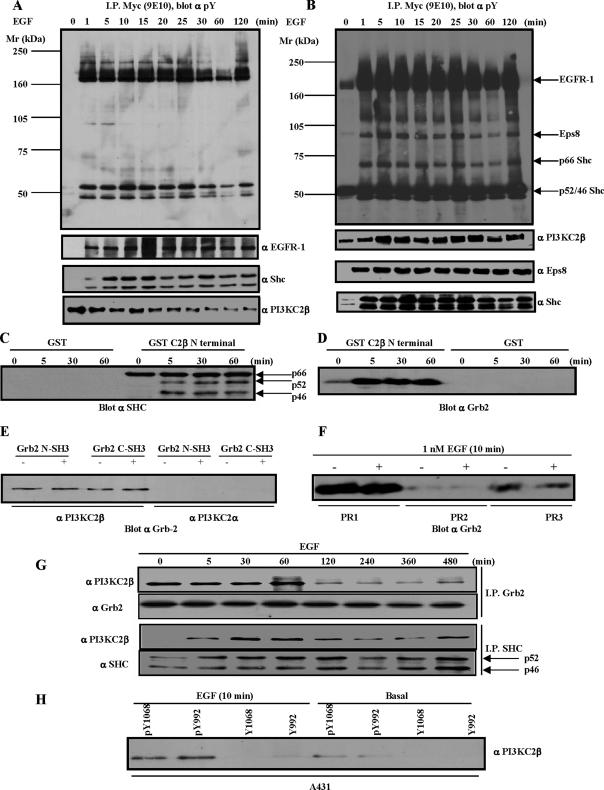
Figure 1.
Identification of PI3KC2β-binding proteins. (A) A-431-C2β WT cells were grown to 80% confluence and serum-starved for 24 h before stimulation with EGF for the times indicated. The Triton X-100–soluble (A) and –insoluble fraction (B) were generated as outlined in Materials and Methods. PI3KC2β-associated proteins were identified by immunoblotting with the indicated antibodies. (C–E) Lysates from parental A-431 cells were equalized for protein content before immunoprecipitation with the indicated GST-fusion proteins. PI3KC2β N-terminal 300 amino acid domain mediates recruitment of Shc and Grb2 (C and D). Gst-Grb2 pulldowns indicate selective recruitment of PI3KC2β (E). (F) A-431 cell lysates were equalized for protein content before immunoprecipitations with the Actigel coupled PI3KC2β proline-rich peptides, PR1, PR2, and PR3. The amount of Grb2 precipitated by the respective peptides was determined by imuunoblotting. (G) The A-431-C2β WT cell line was induced with 1 nM EGF for the times indicated before immunoprecipitation with Shc and Grb2. Analysis of the respective immune-complexes indicates that Grb2 and the p46/p52Shc isoforms both associate with PI3KC2β. (H) A-431 cell lysates were immunoprecipitated with Actigel coupled phosphorylated and nonphosphorylated peptides representing the EGFR-1 SH2-binding peptides for Grb2 (pY1068/Y1068) and Shc (pY992/992). The amount of endogenous PI3KC2β pulled down was determined by immunoblotting.