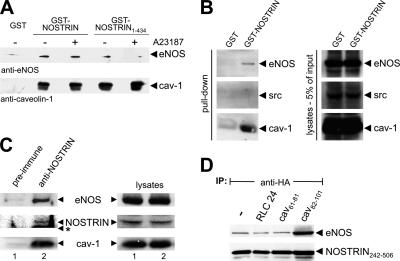
Figure 3.
NOSTRIN, caveolin-1, and eNOS form a ternary complex. The scaffolding domain of caveolin-1 enhances interaction of NOSTRIN and eNOS. (A) GST pull-down from lysates of CHO-eNOS cells using full-size GST-NOSTRIN and GST-NOSTRIN1-434, which lacks the eNOS binding SH3 domain. Addition of the Ca2+ ionophore A23187 abolishes the interaction of eNOS and caveolin-1. (B) Pull-down from lysates of CHO-eNOS using GST-NOSTRIN. (C) Coimmunoprecipitations from HUVECs using rabbit anti-NOSTRIN or preimmune serum for control. Western blots of immunoprecipitates and lysates were done with mouse anti-eNOS, anti-NOSTRIN, and anti-caveolin-1. The asterisk indicates the position of the immunoglobulin heavy chain. (D) Coimmunoprecipitations from CHO-eNOS cells infected with SFV-HA-NOSTRIN242-506 using anti-HA. Caveolin-1 peptides cav82-101 (scaffolding domain), cav61-81, or unrelated peptide (RLC24) were added to the lysates as indicated. Immunoblots were probed for NOSTRIN (anti-HA) and eNOS.