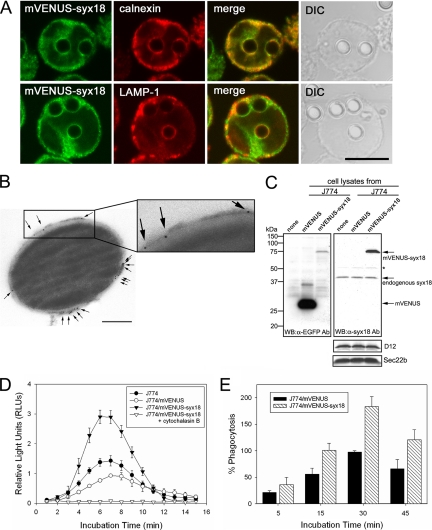
Figure 7.
mVENUS-syntaxin 18 is localized on phagosomal membranes surrounding nonopsonized latex beads and enhances phagocytosis of luminol-bound microbeads in J774 macrophages. (A) J774 cells expressing mVENUS-syntaxin 18 (J774/mVENUS-syx18 cells) were incubated in the presence of nonopsonized latex beads (3.0 μm diameter) for 20 min before fixation. Cells were then stained with antibodies against calnexin or LAMP-1. Bar, 10 μm. (B) Electron microscopic (EM) analysis of mVENUS-syntaxin 18 recruitment on purified phagosomes. Formed phagosomes ingested 0.8-μm diameter blue-dyed latex beads for 20 min and were then purified by sucrose gradient centrifugation from J774/mVENUS-syx18 cells. The purified phagosomes were labeled with an anti-EGFP antibody and a goat anti-rabbit antibody coupled to 5-nm gold (arrows indicate some of the gold particles) as described in Materials and Methods (also see Supplementary Figure S3). Bar, 200 nm. (C) Western blotting analysis of total lysates from J774 cells stably expressing mVENUS and mVENUS-syntaxin 18. Western blotting was carried out using antibodies against EGFP and syntaxin 18. The expression of mVENUS-syntaxin 18 was about threefold higher than that of endogenous syntaxin 18 in J774/mVENUS-syntaxin 18 cells. Asterisk (*) denotes nonspecific band recognized by anti-syntaxin 18 antibodies. The expression levels of D12 and Sec22b in these cells were not different. (D) J774, J774/mVENUS, and J774/mVENUS-syntaxin 18 cells were incubated with luminol-bound microbeads. Chemiluminescence from cells that ingested the beads was measured on a TD-20/20 luminometer for 15 s every 1 min, up to 15 min. These data indicate the rate of phagocytosis in the cells. When J774/mVENUS-syntaxin 18 cells were incubated with the beads in the presence of cytochalasin B (final 10 μM), no significant signal was detected. Data presented are the mean ± SE of six independent experiments. (E) J774/mVENUS and J774/mVENUSsyntaxin 18 cells were incubated with Texas Red-conjugated zymosan A prespectively, as articles. At the indicated time points, samples were washed in PBS to remove free particles and fixed and then processed for fluorometric analyses as described in Materials and Methods (also see Supplementary Figure S5). Arbitrary fluorescence units were obtained by subtracting the average fluorescence intensity in the presence of cytochalasin B from that in the absence of cytochalasin B and then normalizing to the maximal value obtained for J774/mVENUS cells within the same experiment. Data presented are the mean ± SE of three independent experiments.