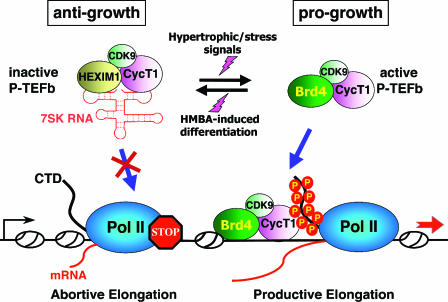
FIG. 5.
P-TEFb is maintained in a functional equilibrium by dynamic associations with its positive and negative regulators. In the nucleus, a major portion of P-TEFb is sequestered into the 7SK/HEXIM1 snRNP, where P-TEFb's kinase activity is inhibited by HEXIM1 in a 7SK-dependent manner. Because P-TEFb within the 7SK snRNP is unable to phosphorylate the Pol II CTD or associate with promoters, Pol II goes into the abortive elongation mode. Treatment of HeLa cells with stress-inducing agents or cardiac myocytes with hypertrophic signals can cause a rapid disruption of the 7SK snRNP and quantitative conversion of the released P-TEFb into the Brd4-bound form. This results in the increased recruitment of P-TEFb by Brd4 to transcriptional templates and stimulation of productive elongation by Pol II. On the other hand, when murine erythroleukemia cells are induced to differentiate by the treatment with HMBA, the P-TEFb equilibrium is shifted to the inactive, HEXIM1/7SK-bound state. Thus, the dynamic associations of P-TEFb with its positive and negative regulators are kept under tight cellular control in response to ever-changing transcriptional demand in the cell. Since HEXIM1 is known to display an antigrowth effect in a number of cell types, whereas Brd4 is progrowth during mouse development, their targeting of the general transcription factor P-TEFb is expected to affect the global control of cell growth and differentiation. For simplicity, only a monomer each of P-TEFb and HEXIM1 is depicted in the 7SK snRNP.