Abstract
Twenty-one intussusception (IS)-associated and 59 temporally linked adenoviral isolates from clinical infections were compared. Species C (15/21 IS- and 32/59 non-IS-associated isolates) dominated. Of these, serotype 2 (AdV-2) (7/15 IS-associated isolates) and serotype 1 (AdV-1) (16/32 non-IS-associated isolates) were the most commonly identified serotypes. DNA restriction analysis of AdV-2 isolates identified six genomic types; of these, type D2 (3/7 IS- and 8/11 non-IS-associated isolates) was the dominant type after BamHI and SmaI digestion. IS-associated isolates are similar to circulating non-IS-associated strains.
Intussusception (IS) is the most common cause of intestinal obstruction among young children, occurring when one segment of the intestine invaginates an adjacent segment of the distal intestine. Progression can culminate in arterial obstruction and subsequent bowel necrosis (6). In the majority of childhood IS cases, no clear etiology is identified, but adenovirus has been isolated in up to 40% of cases by our group and others, with significantly lower isolation rates in healthy controls (5). A rhesus-human reassortant rotavirus vaccine was withdrawn in 1999 in the United States due to a strong temporal association with IS (11).
Human adenoviruses cause a wide spectrum of clinical disease, from uncomplicated acute respiratory and gastrointestinal infections in normal healthy individuals to chronic systemic infection in immunosuppressed hosts. Human adenoviruses are classified into 51 serotypes within six species (A to F). Species C adenoviruses are the most commonly isolated respiratory pathogens, involving AdV-1, AdV-2, and AdV-5 (13).
While an association between nonenteric adenovirus and IS has been reported (4, 7), the virus has not been characterized. It is not known whether IS-associated strains differ from prevalent strains causing symptomatic respiratory or conjunctival illness in young children. In this study, we investigated the temporal distribution and genetic relationship of adenovirus strains isolated from IS patients at the Royal Children's Hospital (RCH), Melbourne, Australia.
Twenty-one adenoviral isolates were obtained prospectively from fecal specimens collected from children less than 2 years of age, admitted to the RCH with IS from January 2003 until December 2004, as previously described (5). IS-associated isolates were detected in both years, including nine IS cases between July and November (winter and spring) 2003, with no similar clustering in 2004. In the week preceding IS, possible symptoms were observed by the parents of 8/20 IS patients. This group included seven patients with respiratory tract infection symptoms and one with fever alone.
During the study period, 198 non-IS-associated adenoviral strains were identified from respiratory, conjunctival, and gastrointestinal specimens sent for routine diagnostic identification at the RCH virology department. Adenoviruses were detected year round with no particular seasonality. Peaks were observed in January and November 2004, with 23 and 20 positive identifications, respectively. Fifty-nine isolates were selected for analysis based upon temporal relationship (within 1 to 4 weeks) with IS-associated isolates, including 32 from children less than 2 years old.
Species typing (A to F) was performed on all 21 IS- and 59 temporally linked non-IS-associated isolates. DNA was extracted from isolates adapted to A549 cells (4) and typed using species-specific primers to the fiber gene (18). Species C was the dominant type for both IS (15/21)- and non-IS (32/59)-associated adenoviruses (P = 0.12, Fisher exact test) (Table 1).
TABLE 1.
Adenovirus species distribution, and species C serotype distribution, of clinical samples (isolated from 2003 to 2004) as determined by species-specific multiplex PCR, hexon gene PCR, and sequence analysis
| Species or serotype | No. of isolates from indicated source
|
||||
|---|---|---|---|---|---|
| IS associatedb | Non-IS associated
|
||||
| Diarrhea | Respiratory | Conjunctival | Total | ||
| A | 1 | 0 | 0 | 0 | 0 |
| B | 1 | 2 | 9 | 4 | 15 |
| C | 15c | 11 | 20 | 1 | 32 |
| AdV-1 | 4 | 2 | 13 | 1 | 16 |
| AdV-2 | 7 | 6 | 5 | 0 | 11 |
| AdV-5 | 4 | 3 | 2 | 0 | 5 |
| D | 0 | 0 | 0 | 7 | 7 |
| E | 1 | 0 | 0 | 0 | 0 |
| F | 2 | 0 | 1 | 2 | 3 |
| NTa | 1 | 0 | 0 | 2 | 2 |
| Total | 21 | 13 | 30 | 16 | 59 |
NT, nontypeable.
All IS-associated isolates were derived from fecal specimens.
Subtotal for AdV-1, AdV-2, and AdV-5 serotypes.
All species C isolates were further analyzed for serotype by PCR and sequence analysis of the hexon gene as described previously (14, 16). For IS-associated isolates, AdV-2 was most frequently identified (7/15), followed by AdV-1 and AdV-5 (4/15 each). For the non-IS-associated isolates, AdV-1 was most commonly identified (16/32), predominantly from respiratory cases (13/20) (Table 1). No AdV-6 isolates were identified.
DNA restriction analysis was used to evaluate the genetic diversity of AdV-2. DNA was digested individually with BamHI and SmaI (New England BioLabs, MA), and restriction patterns were assigned according to the system proposed by Adrian et al. (2, 3).
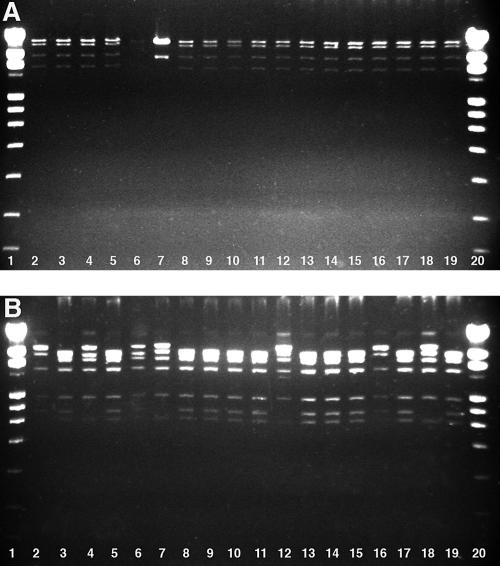
Identical BamHI profiles (represented by four bands) were seen in 17/18 isolates analyzed (Fig. 1). The 18 AdV-2 isolates exhibited five different SmaI profiles, consisting of four to seven bands (Fig. 1). Using combined BamHI and SmaI data, isolates were classified into six different genome types, termed D1 to D6 (Table 2). D2 predominated in both IS- and non-IS-associated strains. Strains isolated from patients with IS were of five different genetic types, with non-IS-associated isolates being of three genetic types.
FIG. 1.
Restriction enzyme patterns of species C AdV-2 isolates obtained from IS (lanes 2 to 7) and non-IS (lanes 8 to 19) cases. Genomic profiles were resolved by 1.2% Tris-borate-EDTA agarose gel electrophoresis. Panel A depicts DNA digested with BamHI, and panel B depicts profiles from SmaI restriction enzyme digestion. Lanes 1 and 20, DNA ladder 1V (Roche, Mannheim, Germany).
TABLE 2.
Genomic classification of AdV-2 isolates obtained from IS and non-IS cases
| Genome type | Enzyme code
|
Mo/yr of isolation | No. of isolates
|
|||
|---|---|---|---|---|---|---|
| BamHI | SmaI | IS | Non-IS | Total | ||
| D1 | 1 | 1 | Aug. 2003 | 1 | 1 | |
| 1 | 1 | Oct. 2003 | 1 | 1 | ||
| D2 | 1 | 2 | Mar. 2003 | 1 | 1 | |
| June 2003 | 2 | 2 | ||||
| Aug. 2003 | 1 | 3 | 4 | |||
| Oct. 2003 | 1 | 1 | ||||
| Feb. 2004 | 1 | 1 | ||||
| Oct. 2004 | 1 | 1 | ||||
| Nov. 2004 | 1 | 1 | ||||
| D3 | 1 | 3 | Sept. 2003 | 1 | 1 | |
| D4 | 1 | 4 | Apr. 2004 | 1 | 1 | |
| D5 | 2 | 5 | July 2004 | 1 | 1 | |
| D6 | 1 | 5 | July 2003 | 2 | 2 | |
| Total | 7 | 11 | 18 | |||
Adenoviruses are an important cause of respiratory infection in infants and children and have been associated with a significant proportion of cases of IS (5, 7, 12, 17). To discern the potential contribution of viral factors in the pathogenesis of IS, we studied the molecular epidemiology and genetic characterization of adenoviruses isolated from children with IS. We compared these isolates to temporally associated adenoviruses isolated from non-IS illness (respiratory, gastroenteritis, and conjunctival) over a 24-month period.
No temporal clustering of IS-associated adenovirus was identified in this study, and genetic characterization did not demonstrate clustering of adenoviral species or serotypes.
Of the 79 adenovirus isolates typed, species C accounted for the majority of IS- and non-IS-associated isolates. Species C is widely represented in most populations, representing more than half of the clinical adenovirus isolations worldwide. In this study, characterization of species C isolates into serotypes identified types AdV-1, AdV-2, and AdV-5 in both IS and non-IS cases, with no significant differences identified between the groups. AdV-6 was absent as also noted in other studies (2, 3).
AdV-2, the most common IS-associated serotype in this study, is most frequently described as a respiratory pathogen but has been associated with gastroenteritis (9). In the routine clinical samples typed, AdV-2 was characterized in 6 of 13 gastroenteritis isolates and only 5 of 30 respiratory isolates. AdV-2 genomic variation was investigated further. Interestingly, five of six genetic types identified were present in IS-associated strains, while only three types were present in the non-IS-associated strains. This large genetic diversity is supported by previous analysis of species C serotypes in non-IS-associated isolates (1, 2). Despite this strain diversity, D2 was shown to be the dominant genome type in both IS- and non-IS-associated adenovirus isolates. This suggests that a common dominant type associated with IS and non-IS clinical disease manifestations is circulating in the community.
Establishment of cause by isolation of adenovirus from IS patients was beyond the scope of our study. The significantly increased isolation rate compared to that of healthy controls in a previous study (5) strongly supports an association. This reduces the likelihood that shedding of adenovirus in IS patients is coincidental. It has been speculated that adenoviral infection may predispose infants to development of acute IS. Idiopathic cases are believed to arise from hypertrophy of infected lymph nodes, including Peyer's patches acting as physical “lead points” in the pathogenesis of IS, although pathological studies do not consistently identify hypertrophied lymphatic tissue (8, 10). If adenoviral infection is in fact a precursor to IS, it can be hypothesized from our findings that susceptible children will acquire commonly circulating community strains. Given that most young children infected with the adenovirus suffer only mild illness, such as respiratory infection, we can further speculate that susceptible individuals may have an altered anatomic or immune status that, when they are infected with adenovirus, predisposes them to IS. This hypothesis is consistent with the susceptible-host hypothesis stemming from the Rotashield experience, where a small number of cases occurred despite widespread vaccine uptake (15). Host immune response may be the key determinant of the clinical course of infection after adenovirus colonization. Evaluation of host responses to adenovirus, together with exploration of adenoviral virulence determinants, remains a priority area of investigation into the most common cause of acute bowel obstruction in young children.
Acknowledgments
We are grateful for the technical assistance of Robert Alexander and laboratory members from the Virology Department, Royal Children's Hospital, as well as Nada Bogdanovic and Fran Justice from the Enteric Virus Group, Murdoch Children's Research Institute.
This work was supported by a project grant from the Murdoch Children's Research Institute, Melbourne, Australia.
REFERENCES
- 1.Adrian, T. 1996. Genome polymorphism of human adenoviruses of subgenus C. Arch. Virol. 141:1021-1031. [DOI] [PubMed] [Google Scholar]
- 2.Adrian, T., B. Best, and R. Wigand. 1985. A proposal for naming adenovirus genome types, exemplified by adenovirus type 6. J. Gen. Virol. 66:2685-2691. [DOI] [PubMed] [Google Scholar]
- 3.Adrian, T., J. Sassinek, and R. Wigand. 1990. Genome type analysis of 480 isolates of adenovirus types 1, 2, and 5. Arch. Virol. 112:235-248. [DOI] [PubMed] [Google Scholar]
- 4.Bhisitkul, D. M., K. M. Todd, and R. Listernick. 1992. Adenovirus infection and childhood intussusception. Am. J. Dis. Child. 146:1331-1333. [DOI] [PubMed] [Google Scholar]
- 5.Bines, J. E., T. N. Liem, F. A. Justice, T. N. Son, C. D. Kirkwood, M. De Campo, P. Barnett, R. F. Bishop, R. Robins-Browne, and J. B. Carlin. Risk factors for intussusception in Vietnam and Australia: adenovirus implicated, not rotavirus. J. Pediatr., in press. [DOI] [PubMed]
- 6.DiFiore, J. W. 1999. Intussusception. Semin. Pediatr. Surg. 8:214-220. [DOI] [PubMed] [Google Scholar]
- 7.Hsu, H. Y., C. L. Kao, L. M. Huang, Y. H. Ni, H. S. Lai, F. Y. Lin, and M. H. Chang. 1998. Viral etiology of intussusception in Taiwanese childhood. Pediatr. Infect. Dis. J. 17:893-898. [DOI] [PubMed] [Google Scholar]
- 8.Kombo, L. A., M. A. Gerber, L. K. Pickering, C. D. Atreya, and R. F. Breiman. 2001. Intussusception, infection, and immunization: summary of a workshop on rotavirus. Pediatrics 108:E37. [DOI] [PubMed] [Google Scholar]
- 9.Li, L., T. G. Phan, T. A. Nguyen, K. S. Kim, J. K. Seo, H. Shimizu, E. Suzuki, S. Okitsu, and H. Ushijima. 2005. Molecular epidemiology of adenovirus infection among pediatric population with diarrhea in Asia. Microbiol. Immunol. 49:121-128. [DOI] [PubMed] [Google Scholar]
- 10.Lynch, M., W. J. Shieh, J. S. Bresee, K. M. Tatti, J. R. Gentsch, T. Jones, B. Jiang, E. Hummelman, C. M. Zimmerman, S. R. Zaki, and R. I. Glass. 2006. Intussusception after administration of the rhesus tetravalent rotavirus vaccine (Rotashield): the search for a pathogenic mechanism. Pediatrics 117:e827-e832. [DOI] [PubMed] [Google Scholar]
- 11.Murphy, T. V., P. M. Gargiullo, M. S. Massoudi, D. B. Nelson, A. O. Jumaan, C. A. Okoro, L. R. Zanardi, S. Setia, E. Fair, C. W. LeBaron, M. Wharton, and J. R. Livengood. 2001. Intussusception among infants given an oral rotavirus vaccine. N. Engl. J. Med. 344:564-572. [DOI] [PubMed] [Google Scholar]
- 12.Nicolas, J. C., D. Ingrand, B. Fortier, and F. Bricout. 1982. A one-year virological survey of acute intussusception in childhood. J. Med. Virol. 9:267-271. [DOI] [PubMed] [Google Scholar]
- 13.Schmitz, H., R. Wigand, and W. Heinrich. 1983. Worldwide epidemiology of human adenovirus infections. Am. J. Epidemiol. 117:455-466. [DOI] [PubMed] [Google Scholar]
- 14.Shinagawa, M., A. Matsuda, T. Ishiyama, H. Goto, and G. Sato. 1983. A rapid and simple method for preparation of adenovirus DNA from infected cells. Microbiol. Immunol. 27:817-822. [DOI] [PubMed] [Google Scholar]
- 15.Smith, P. J., B. Schwartz, A. Mokdad, A. B. Bloch, M. McCauley, and T. V. Murphy. 2003. The first oral rotavirus vaccine, 1998-1999: estimates of uptake from the National Immunization Survey. Public Health Rep. 118:134-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeuchi, S., N. Itoh, E. Uchio, K. Aoki, and S. Ohno. 1999. Serotyping of adenoviruses on conjunctival scrapings by PCR and sequence analysis. J. Clin. Microbiol. 37:1839-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Velazquez, F. R., G. Luna, R. Cedillo, J. Torres, and O. Munoz. 2004. Natural rotavirus infection is not associated to intussusception in Mexican children. Pediatr. Infect. Dis. J. 23:S173-S178. [DOI] [PubMed] [Google Scholar]
- 18.Xu, W., M. C. McDonough, and D. D. Erdman. 2000. Species-specific identification of human adenoviruses by a multiplex PCR assay. J. Clin. Microbiol. 38:4114-4120. [DOI] [PMC free article] [PubMed] [Google Scholar]