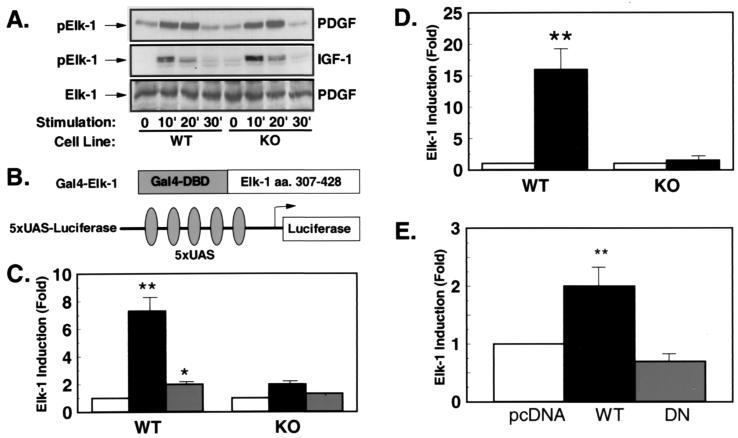
Figure 4.
PDGF- and IGF-1-stimulated transcriptional activity of Elk-1 is reduced in the presence of unaltered Elk-1 phosphorylation. (A) Western blot analyses were performed with an anti-Elk-1 antibody (Bottom) or an phospho-specific anti-Elk-1 antibody (Top and Middle) on cell extracts of PDGF- (Top and Bottom) or IGF-1-treated (Middle) cells. (B) Schematic representation of the heterologous Elk-1 reporter system. (C) WT and KO cells were cotransfected with the described reporter plasmid, Gal-4–Elk-1 and the plasmid PEF–β-galactosidase (PEF–β-gal) expressing β-galactosidase under control of the elongation factor promoter. After culture in serum-free medium for 36 hr, cells were left untreated (white bars) or treated with PDGF (black bars) or IGF-1 (gray bars) for 4 hr before lysis. Data represent the stimulation of luciferase activity/β-galactosidase activity in stimulated vs. untreated cells. Data are the mean of four independent experiments ± SEM. (*, P < 0.05; **, P < 0.01). (D) Cotransfections were performed with the described Elk-1-reporter in the presence of pcDNA or a MEK-1-expression plasmid (black bars) (**, P < 0.01). (E) KO cells were transfected with 5XUAS-luciferase and Gal-4–ElkC together with the expression plasmid pcDNA (white bar), RSV-WTRsk (black bar), or RSV-DNRsk (gray bar) expressing wild-type Rsk (WTRsk) or a dominant–negative mutant of Rsk-2 (DNRsk). Data presented are the mean of four experiments ± SEM expressed as stimulation compared with pcDNA-transfected cells (**, P < 0.01).