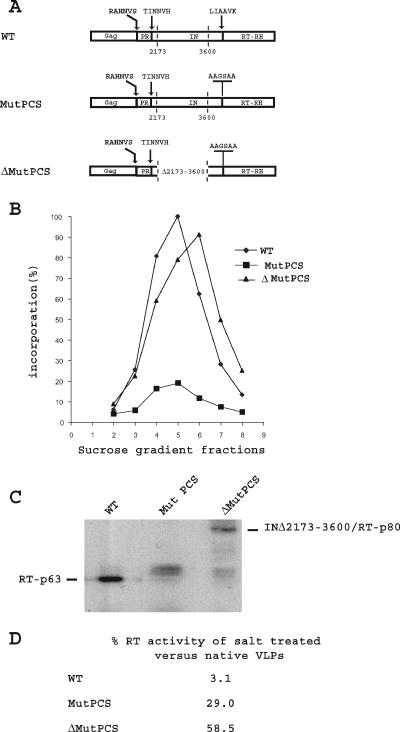
FIG. 3.
(A) Schematic representation of the Gag-Pol polyprotein of the WT element, the IN/RT protease cleavage site (PCS), mutant element and the IN deletion mutant element bearing an IN/RT PCS mutation. The sequences of the Gag/IN, PR/IN, IN/RT, and mutant IN/RT PCS are indicated. The endpoints of the deletion are indicated by vertical dotted lines at positions 2173 and 3600. These numbers are the Ty1-H3 positions (2) and correspond to amino acid residues 45 and 521 of the IN protein, which contains 635 amino acids. (B) VLPs were prepared from cells expressing these elements and purified on a sucrose step gradient. RT activity was assayed using an oligo(dG)/poly(rC) primer template. 100% incorporation is the label incorporated in the peak fraction (fraction 5) of WT VLPs. It is equal to 3 to 7 pmol of dGTP incorporated in 30 min for 4 μl of sucrose gradient-purified VLPs. (C) Immunoblot analysis. The volumes of VLPs that were found to incorporate the same amount of dGTP into high-molecular-mass poly(dG) by the oligo(dG)/poly(rC) primer template assay were loaded onto the gel and probed with Ty1 RT antibodies. (D) The RT activity of the native or salt-treated VLPs was tested on an exogenous primer-template. The percent activities of salt-treated VLPs relative to native VLPs are indicated.