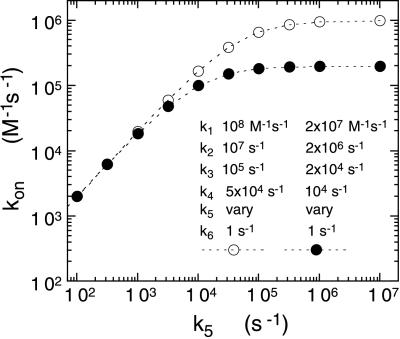
Figure 5.
Calculated relationship of the value of kon (e.g., the second order rate constant for reaction with sulfite) as a function of the value of k5 for the model described in the text. The values of kon were calculated from the ratio k1k3k5/(k2k4 + k2k5 + k3k5) as described in the text. The same values were confirmed by computer simulation of the three-step binding model. The two sets of curves simulate a 5-fold viscosity effect on the entry and positioning of reactant before the observed chemical step, k5.